Nephrology 1
Session: Nephrology 1
002 - First-year outcomes of pediatric kidney transplant recipients stratified by absolute CD3+ cell counts following rabbit anti-thymocyte globulin induction immunosuppression
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 2.4912
Travis Churilla, Ann & Robert H. Lurie Children's Hospital of Chicago, Chicago, IL, United States; Priya S. Verghese, Ann & Robert H. Lurie Children's Hospital of Chicago, Chicago, IL, United States; Natalia Panek, Ann & Robert H. Lurie Children's Hospital of Chicago, Wilmette, IL, United States

Travis Churilla, MD (he/him/his)
Pediatric Nephrology Fellow, PGY-4
Ann & Robert H. Lurie Children's Hospital of Chicago
Chicago, Illinois, United States
Presenting Author(s)
Background: Absolute CD3+ cell count can determine the degree of active T-cell depletion, but what threshold of this depletion appropriately balances the risks and benefits of rabbit anti-thymocyte globulin (rATG) administration is uncertain.
Objective: We aimed to test for an association between the absolute CD3+ cell count used to guide rATG induction dosing and subsequent clinical outcomes in pediatric kidney transplant recipients.
Design/Methods: Pediatric kidney transplant recipients transplanted between 2020-2024 were included for retrospective medical record review if they met the following criteria: (1) < 21 years of age at transplantation, (2) no previous receipt of a solid organ or hematopoietic stem cell transplant, (3) completion of a rATG-based induction protocol, (4) had absolute CD3+ T-cell counts quantified via flow cytometry at induction, and (5) at least 6 months post-transplant follow-up. An absolute CD3+ cell count cutoff of < 25 (vs. 25) for grouping was defined based on our current protocol. Patients were grouped by their CD3+ cell counts at time of flow cytometry irrespective of whether it was obtained after 2 or 3 doses of rATG. Relevant pre-transplant data was obtained. Outcome data was collected for variables pertaining to infections, hospitalizations, and graft rejection/survival. Statistical analyses were performed using R (version 4.3.2).
Results: Of 132 records queried, 28 patients met criteria for enrollment with a median age of 15 years (IQR = 13.75-16.62). A total of 28 patients had data available for at least 6-months post-transplant, 23 of whom had a 12-month follow-up. In this cohort, 78.5% (22/28) of patients had an absolute CD3+ count < 25 when checked following administration of either two (9/10 = 90%) or three (13/18 = 78%) doses of rATG. The cumulative dose, in mg/kg, received prior to flow cytometry did not differ between those that achieved a CD3+ count < 25 and those that did not (4.35 vs. 4.24, p = 0.700). The rates of rejection at 6-months (18.18% vs. 16.67%, p = 0.831) and 12-months (33.33% vs. 40%, p = 0.836) were not significantly different. The incidence of viral-DNAemia or urinary traction infections, did not differ between groups at 6- and 12-months. The rates and cumulative length of stay of hospitalizations (infections and all-cause) at these endpoints also did not differ. There was no graft loss or mortality.
Conclusion(s): First year post-transplant outcomes did not differ between patients with CD3+ absolute cell counts < 25 vs. >= 25, suggesting that routine surveillance of the CD3+ cell counts during kidney transplantation may not be clinically relevant.
Confirmation of Trainee Status (Fellow, Pediatric Nephrology)
Fellowship Attestation.pdf
Table 2. Comparison of outcomes at 6-months post-transplant for pediatric kidney transplant recipients based on absolute CD3+ cell count following rabbit anti-thymocyte globulin induction (n = 28)
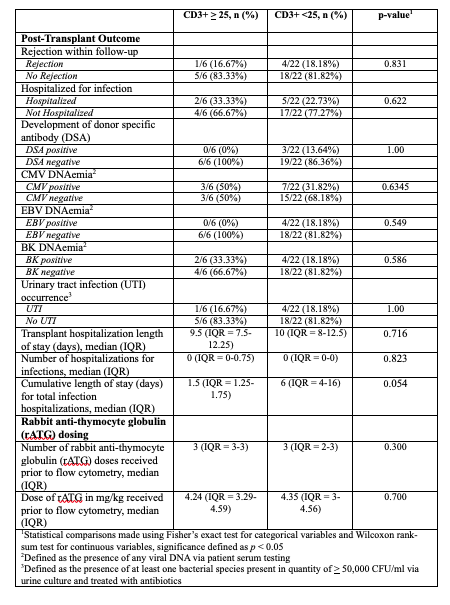 1Statistical comparisons made using Fisher’s exact test for categorical variables and Wilcoxon rank-sum test for continuous variables, significance defined as p < 0.05
1Statistical comparisons made using Fisher’s exact test for categorical variables and Wilcoxon rank-sum test for continuous variables, significance defined as p < 0.052Defined as the presence of any viral DNA via patient serum testing
3Defined as the presence of at least one bacterial species present in quantity of > 50,000 CFU/ml via urine culture and treated with antibiotics
Table 3. Comparison of cumulative outcomes by 12-months post-transplant for pediatric kidney transplant recipients based on absolute CD3+ cell count following rabbit anti-thymocyte globulin induction (n = 23)
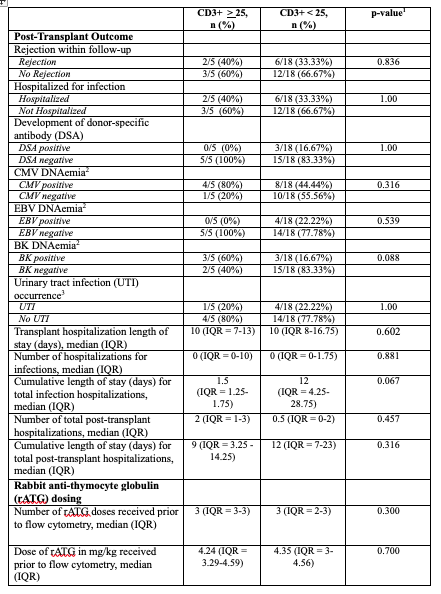 1Statistical comparisons made using Fisher’s exact test for categorical variables and Wilcoxon rank-sum test for continuous variables, significant defined as p < 0.05
1Statistical comparisons made using Fisher’s exact test for categorical variables and Wilcoxon rank-sum test for continuous variables, significant defined as p < 0.052Defined as the presence of any viral DNA via patient serum testing
3Defined as the presence of at least one bacterial species present in quantity of > 50,000 CFU/ml via urine culture and treated with antibiotics

