Neonatal Neurology 4
Session: Neonatal Neurology 4
647 - Incidence and outcomes for infants treated with therapeutic hypothermia after hypoxic-ischemic encephalopathy: a national population-based study
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 647.5290
Karoline Aker, Norwegian University of Science and Technology, Trondheim, Sor-Trondelag, Norway; Guro L Andersen, Vestfold Hospital Trust, Tønsberg, Vestfold, Norway; Mari Falck, Oslo University Hospital, Oslo, Oslo, Norway; Claus Klingenberg, University Hospital Of North Norway, Tromsø, Troms, Norway; Dag Moster, Haukeland University Hospital, Bergen, Hordaland, Norway; Arild E Rønnestad, University of Oslo, Oslo, Oslo, Norway; Hans Jørgen Stensvold, Oslo University Hospital, Oslo, Oslo, Norway; Janicke Syltern, St. Olavs hospital, Trondheim, Sor-Trondelag, Norway; Ragnhild Støen, St. Olav hospital Trondheim University hospital, TRONDHEIM, Sor-Trondelag, Norway

Karoline Aker, M.D, PhD (she/her/hers)
Postdoctoral fellow
Norwegian University of Science and Technology
Trondheim, Sor-Trondelag, Norway
Presenting Author(s)
Background: Therapeutic hypothermia (TH) was implemented in Norway in 2007 and is still the only neuroprotective treatment available in routine clinical care for infants with moderate to severe hypoxic-ischemic encephalopathy (HIE).
Objective: To assess the use of TH and preschool outcomes of cooled infants with HIE in a national cohort of term and near-term born infants. The secondary objective was to explore regional differences.
Design/Methods: This was a population-based study of all infants in Norway born at or near term (gestational age ≥36+0 weeks) who received TH between 2009 and 2016. The study is based on data from five Norwegian national health registries; the Norwegian Neonatal Network, the Medical Birth Registry of Norway, the Norwegian Patient Registry, the Cause of Death Registry, and the Norwegian Quality and Surveillance Registry for Cerebral Palsy. Preschool outcomes (death or ICD-10 diagnoses of cerebral palsy (CP), epilepsy, hearing impairment requiring hearing aids, and intellectual disability) were collected at 5-6 years of age.
Results: During the study period, 486,412 live born infants in Norway, and 497 (1.02 per 1000 live born infants) received TH (figure 1). Neonatal characteristics and outcomes are presented in table 1. One-third of infants had an amplitude-integrated electroencephalography (aEEG) registration documented before initiation of TH, and 42/161 (26%) of those with aEEG results available had normal background activity (table 2). In total, 72/497 (15%) of infants treated with TH died and 44/425 (10%) of survivors had CP (table 1). Among 381 survivors without CP 10 (3%) had epilepsy, 9 (2%) had hearing impairment requiring hearing aid, and one (0.3%) had intellectual disability.
There was a significant difference in the use of TH between the four Norwegian health regions (1.01 per 1000 live born infants in Region 1, 0.78 in Region 2, 1.29 in Region 3 and 1.39 in Region 4 (p-value 0.0009, figure 1)). The region with the lowest use of TH had a higher proportion of infants with a 10-minute Apgar score ≤5 and the highest mortality rate (table 2).
Conclusion(s): The use of TH in Norway was slightly higher than in a population-based study from Sweden, but lower than reported in England and Wales. The reported use of aEEG was low and a substantial proportion had normal background activity. The combined outcome of death or CP at 23% was comparable to other recent clinical studies. Regional differences in the use of TH decreased over time.
Figure 1: The use of therapeutic hypothermia per 1000 live born infants by year and health region
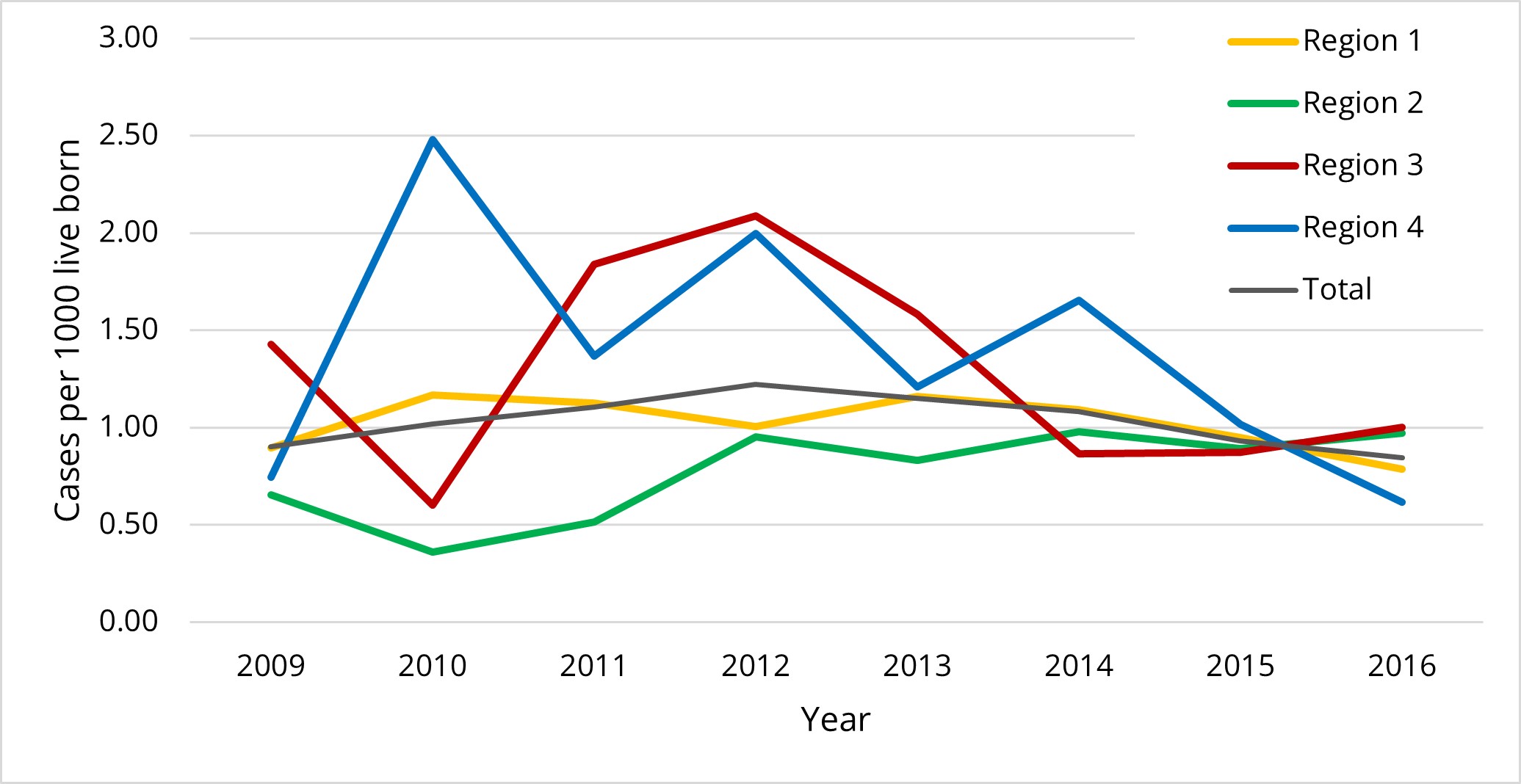
Table 1: Neonatal characteristics and outcomes at 5-6 years of age in infants treated with therapeutic hypothermia (TH)
.jpg) Numbers are n/N (%) unless otherwise indicated. GMFCS: Gross Motor Function Classification System.
Numbers are n/N (%) unless otherwise indicated. GMFCS: Gross Motor Function Classification System.a: 10 infants had missing data.
b: 4 infants had missing data.
c: 208 infants had missing data.
d: 242 infants had missing data.
e: 8 infants had missing data.
Table 2: Neonatal characteristics and outcomes at 5-6 years of age per health region
.jpg) Numbers are n/N (%) unless otherwise indicated. aEEG: amplitude-integrated electroencephalography.
Numbers are n/N (%) unless otherwise indicated. aEEG: amplitude-integrated electroencephalography.a: Metabolic acidosis defined as pH <7.0 or base deficit ≥16.

