Nephrology 4
Session: Nephrology 4
603 - Description of Nephrotoxic Medication Exposure among Critically Ill Children who develop Acute Kidney Injury.
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 603.4436
Daniel S. Thomson, University of Colorado School of Medicine, Denver, CO, United States; John Brinton, University of Colorado School of Medicine, Aurora, CO, United States; Sarah Nelson-Taylor, University of Colorado School of Medicine, Aurora, CO, United States; Erin K. Stenson, University of Colorado School of Medicine, Aurora, CO, United States; Weiwen V. Shih, University of Colorado School of Medicine, Aurora, CO, United States; Sharon Gordon, Children's Hospital Colorado, Aurora, CO, United States

Daniel S. Thomson, MD (he/him/his)
Pediatric Resident
University of Colorado School of Medicine
Denver, Colorado, United States
Presenting Author(s)
Background: In patients admitted to the pediatric intensive care unit (PICU), the development of acute kidney injury (AKI) is independently associated with increased morbidity and mortality. The Nephrotoxic Injury Negated by Just-in-Time Action (NINJA) program is a multicenter quality improvement initiative that screens patients at risk for nephrotoxin-mediated AKI (NTMx-AKI) and has demonstrated a sustained reduction in NTMx-AKI.
Objective: The purpose of this study was to provide a comprehensive analysis of nephrotoxic medications implicated in PICU patients that develop NTMx-AKI and are identified by NINJA.
Design/Methods: This study was a retrospective analysis of patients admitted between January 1st, 2019 and December 31st, 2022 to the PICU at Children’s Hospital Colorado who developed NTMx-AKI as identified by the NINJA program. Nephrotoxic medication exposures (NTMx) were compared between patients that developed serum creatinine based KDIGO (Kidney Disease – Improving Global Outcomes) stage 1 AKI vs. severe (stage 2 or 3) AKI. Review of NTMx data indicated exposure to one or more specific NTMx groups (vancomycin, intravenous contrast, antivirals, NSAIDs, calcineurin inhibitors, and piperacillin/tazobactam) between 3 days prior and 7 days after NINJA flag. Mann-Whitney and Chi-square tests compared outcomes for each NTMx group. A Cochrane-Mantel-Haensel Test for nonzero correlation assessed the correlation between supratherapeutic vancomycin or calcineurin inhibitor (tacrolimus, cyclosporine) levels and stage of AKI.
Results: Overall, 72 patients were included in this review (table 1). Vancomycin exposure occurred in 78% of patients who developed NTMx-AKI. Antiviral medications (acyclovir, ganciclovir, valganciclovir, and valacyclovir) were significantly associated with development of severe AKI (stage 1 = 26% vs severe = 55%, p=0.02). Compared to patients with stage 1 AKI, patients with severe AKI had longer ICU length of stay (median 7 vs. 16 days, p=0.007) and a trend towards increased mortality (10% vs 27%, p=0.11). Further, supratherapeutic vancomycin levels were not strongly predictive of severe AKI (43% vs 47%, n=56; p= 0.56) while supratherapeutic calcineurin inhibitor levels trended towards increased development of severe AKI (44% vs. 88%, n=16; p= 0.07).
Conclusion(s): When utilizing the NINJA program in critically ill children, attention should be paid to use of antiviral medications and supratherapeutic levels of calcineurin inhibitors which were associated with severe AKI in our population.
Rising PL3 Promotion-2.pdf
Number of Patients by NTMx exposure and AKI Stage
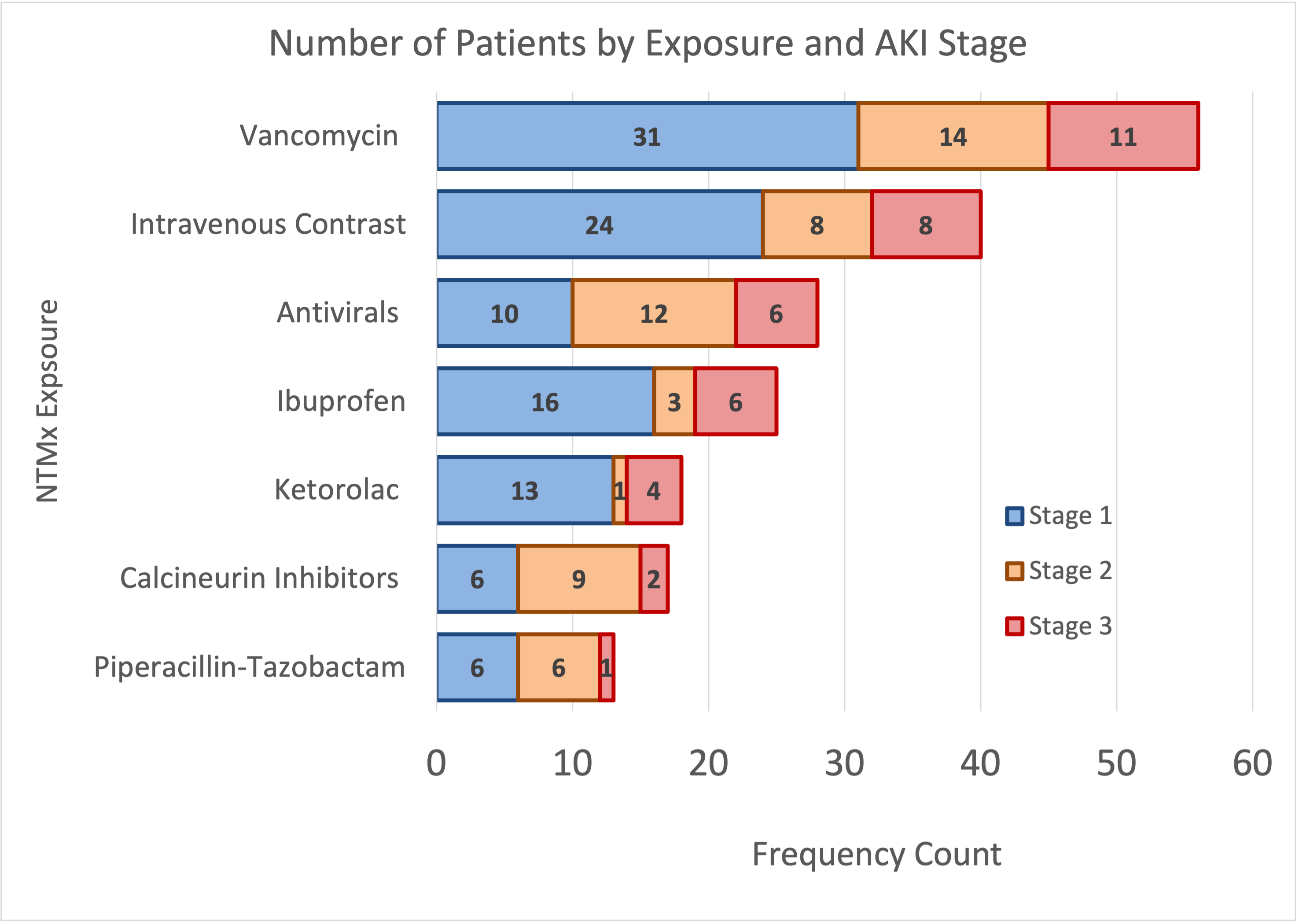 All NTMx exposures are grouped, showing total number of patients that were exposed to that specific medication group and patient AKI stage. Calcineurin inhibitors include tacrolimus and cyclosporine, antiviral medications include acyclovir, ganciclovir, valganciclovir, and valacyclovir.
All NTMx exposures are grouped, showing total number of patients that were exposed to that specific medication group and patient AKI stage. Calcineurin inhibitors include tacrolimus and cyclosporine, antiviral medications include acyclovir, ganciclovir, valganciclovir, and valacyclovir.
