Neonatal GI Physiology & NEC 2
Session: Neonatal GI Physiology & NEC 2
720 - Changes in use of Probiotics in Very Low Birth Weight Infants in the United States from 2016-2024
Saturday, April 26, 2025
2:30pm - 4:45pm HST
Publication Number: 720.4445
Darren Handler, Pediatrix Medical Group, Sunrise, FL, United States; Jennifer M. Canvasser, Necrotizing Enterocolitis (NEC) Society, Davis, CA, United States; Robert Ursprung, Pediatrix Medical Group, Fort worth, TX, United States; Kaashif A. Ahmad, Pediatrix Medical Group, Houston, TX, United States; Ravi M. Patel, Emory University & Children's Healthcare of Atlanta, Atlanta, GA, United States; Veeral Tolia, Pediatrix, Dallas, TX, United States; Rachel G. Greenberg, Duke Clinical Research Institute, Durham, NC, United States

Veeral Tolia, MD
Dir, Clinical Data Warehouse Research
Pediatrix
Dallas, Texas, United States
Presenting Author(s)
Background: Probiotics are live microorganisms that, when administered in adequate amounts, confer a health benefit. A previous study that evaluated the use of probiotics in United States NICUs from 1997 to 2016 found that their usage was increasing and also associated with a lower risk of necrotizing enterocolitis and death. In May 2021, the American Academy of Pediatrics released a statement indicating that “current evidence does not support the routine, universal administration of probiotics to preterm infants.” Additionally, in October 2023, U.S. Food and Drug Administration issued a warning about the risk of invasive disease in preterm infants administered probiotics.
Objective: To describe the recent use of probiotics in a large cohort of very preterm infants focusing on the time period before and after these two significant events.
Design/Methods: The cohort included all infants with a birthweight of 1500 grams or less who were discharged between January 2017 and October 2024 in the Pediatrix Clinical Data (CDW) Warehouse. No infants were excluded from the study. Data for each infant were captured until discharge, death, or transfer to another hospital. Probiotic exposure was defined by the use of “probiotic” or any of the following probiotic formulations (ABC Dophilus, Culturelle, Enfamil infant probiotic, Envivo, Florababy, lactobacillus acidophilus, ProBiota, Tri-blend). Probiotic supplementation was summarized by quarter and year. To maintain deidentification, all dates in the CDW correspond to the date of each infant’s discharge. This may result in some lag in reflecting trends in exposures, particularly for infants with longer hospital stays.
Results: We evaluated 81,315 VLBW infants, of whom 15,904 (18.6%) received probiotics. Probiotic use increased from 13.9% in 2017 to 29.7% in 2023 (figure 1). Overall, there was substantial inter-center variability in use, with 50% of centers reporting < 1% probiotic use in this group of infants. There was minimal change in probiotic use after the 2021 AAP Clinical Report (figure 2). However, probiotic use rapidly declined after the FDA’s warning letter in 2023 from ~30% to < 1% of VLBW infants (figure 3).
Conclusion(s): In this large cohort of VLBW infants cared for in US NICUs, probiotic use increased through 2023 and then dramatically decreased after the warning letter from the FDA. How these recent reductions in probiotic exposure may influence the incidence of necrotizing enterocolitis and mortality remains an area of specific interest. We expect to complete that analysis after the 2024 data collection is complete and have it available for PAS 2025.
Figure 1. Probiotics Exposure by Year 2017-2023
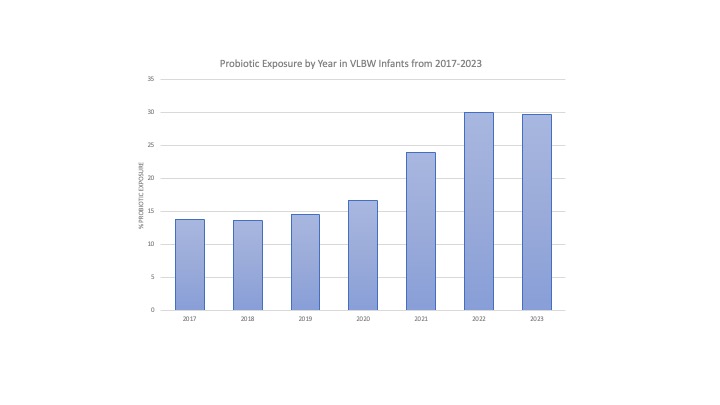
Figure 2. Probiotics Exposure before and after the AAP Statement
.jpg)
Figure 3. Probiotics Exposure before and after the FDA Warning Letter
.jpg)

