Neonatal Quality Improvement 5
Session: Neonatal Quality Improvement 5
525 - Surfactant Delivery in Newborns ≥1250 Grams with Respiratory Distress Syndrome Using Laryngeal Mask Airway: A Quality Improvement Initiative
Saturday, April 26, 2025
2:30pm - 4:45pm HST
Publication Number: 525.6879
Somaya Abuelazm, Cleveland Clinic Children's, Cleveland, OH, United States; Sarah Foley, Cleveland Clinic Children's, Independence, OH, United States; Abdul Kader S. Surti, Cleveland Clinic Children's, Lorain, OH, United States; Sabine Iben, Cleveland Clinic Children's, Cleveland, OH, United States; Firas Saker, Cleveland Clinic Children's, Cleveland, OH, United States; Anna Crist, Cleveland Clinic Children's, Cleveland Heights, OH, United States

somaya abuelazm, MD,FAAP (she/her/hers)
NPM fellow
Cleveland Clinic Children's
Cleveland, Ohio, United States
Presenting Author(s)
Background: Respiratory distress syndrome (RDS) is a significant cause of morbidity and mortality in preterm infants, particularly those with lower birth weights. Surfactant administration using Intubation-Surfactant-Extubation (InSurE) and Less Invasive Surfactant Administration (LISA), require invasive airway intervention. Surfactant Administration Through Laryngeal or Supraglottic Airways (SALSA) offers a non-invasive alternative with the potential to improve outcomes. This quality improvement (QI) project aims to evaluate the efficacy of implementing SALSA in our Neonatal Intensive Care Unit (NICU) for newborns weighing ≥1250 grams with RDS
Objective: To increase the rate of surfactant administration via laryngeal mask airway (LMA) from 0% to 25% by June 2024 and to 50% by June 2025 and to reduce intubation rates by 5% from baseline in newborns weighing ≥1250 grams with RDS
Design/Methods: This prospective QI project included infants ≥1250 grams diagnosed with RDS and requiring surfactant therapy. Eligibility required infants to be on CPAP or NIPPV with radiographic evidence of RDS, FiO₂ >0.40, and PEEP of 6 cm H₂O to maintain oxygen saturations. Exclusions were birth weight < 1250 grams, age < 30 minutes or >48 hours, and previous intubation for transport. The SALSA protocol was established as the first-line method. Process measures tracked adherence to the SALSA protocol, while balancing measures monitored physiological stability during the procedure. Data were collected from July 2023 to September 2024, with Plan-Do-Study-Act (PDSA) cycles guiding interventions and tracking progress
Results: Thirty-two infants received surfactant via LMA (4 in PDSA cycle 1, 6 in cycle 2, 13 in cycle 3, and 16 in cycle 4). The mean gestational age was 33.3 ± 2.4 weeks, with a mean birth weight of 2306.1 ± 818.5 grams. Surfactant was administered at an average age of 22.1 hours with a mean FiO₂ of 0.51 ± 0.14. Surfactant administration via LMA increased from 0% at baseline to 37.5% by the end of PDSA cycle 4. Preliminary data indicate reduced intubation rates with no notable increase in adverse events such as hypoxia, bradycardia, or pneumothorax
Conclusion(s): SALSA implementation in our NICU has effectively increased non-invasive surfactant administration for infants ≥1250 grams with RDS. This QI initiative suggests SALSA as a feasible and safe alternative, potentially reducing intubation rates
figure 1
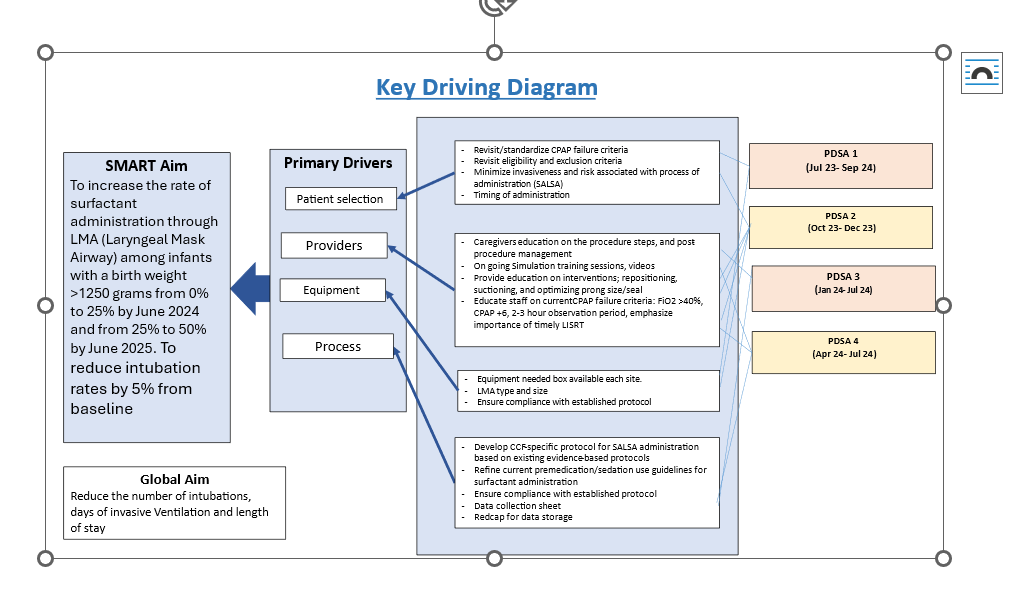 key driver diagram
key driver diagram Figure 2
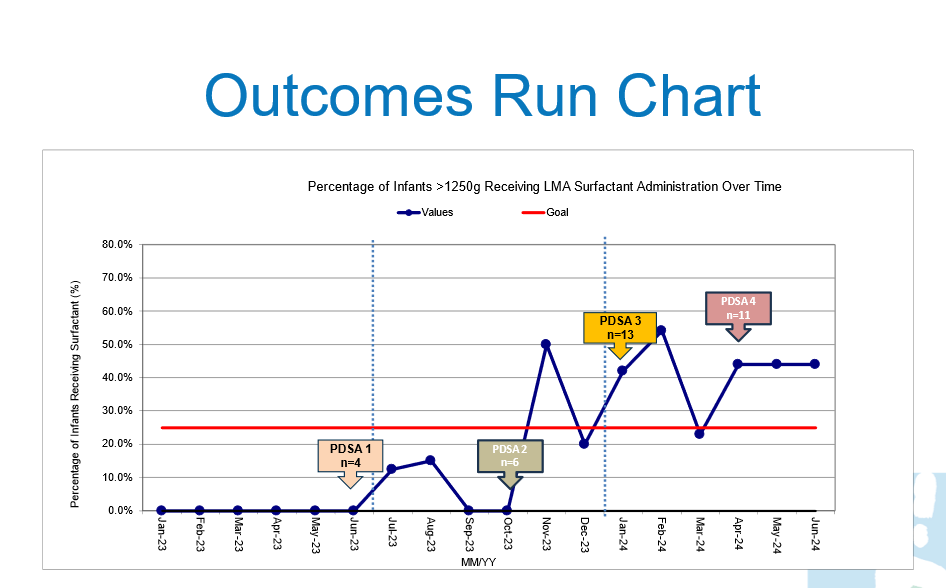 outcome Run Chart, surfactant administration rates via laryngeal mask airway
outcome Run Chart, surfactant administration rates via laryngeal mask airway 

