Emergency Medicine 12
Session: Emergency Medicine 12
536 - An emergency department-based buprenorphine induction and referral program for youth with opioid use disorder
Monday, April 28, 2025
7:00am - 9:15am HST
Publication Number: 536.3638
Gabriel Devlin, Children's Hospital Los Angeles, Los Angeles, CA, United States; Nerininfa Bernabe, Children's Hospital Los Angeles, Moreno Valley, CA, United States; Irene M.. Lim, Children's Hospital Los Angeles, Los Angeles, CA, United States; Alan L.. Nager, CHLA, Los Angeles, CA, United States

Gabriel Devlin, MD/CM (he/him/his)
Pediatric Emergency Medicine Fellow
Children's Hospital Los Angeles
Los Angeles, California, United States
Presenting Author(s)
Background: Recreational opioid use has increased among adolescents. Emergency Departments (EDs) have become critical access points for connecting adults with opioid use disorder (OUD) to medications for addiction treatment (MAT). Whether this is also feasible in pediatric care settings is unknown.
Objective: To describe the characteristics and outcomes of a pilot buprenorphine induction and referral program for pediatric patients presenting to the ED with opioid use disorder
Design/Methods: We developed an ED buprenorphine induction protocol and referral program based on the available literature and multidisciplinary expert opinion (Figure 1). All patients also completed the Emergency Department Distress Response Screener (ED-DRS) and Screening to Brief Intervention (S2BI) tools to assess for additional mental health and psychosocial risk factors. All 44 ED attending physicians received training on the protocol. We implemented the program on July 18, 2023. After a 13-month pilot period, we performed a retrospective chart review of all patients who presented to the ED who were prescribed buprenorphine in the ED or upon discharge. Outcomes of interest included clinical outcomes in the ED, disposition, and 30 day engagement in OUD treatment after discharge.
Results: We identified 11 ED encounters where patients received buprenorphine, of which 73% were in ages 15-17 (Figure 2A). 2 patients presented twice. 73% of patients presented with mild withdrawal, while 27% presented with moderate withdrawal (Figure 3A). Almost all patients reported comorbid depressive symptoms (78%), anxiety (89%), and polysubstance use (89%) (Figure 2B). 67% reported additional psychosocial risk factors (Figure 2B). Most patients only required 1 dose of buprenorphine in the ED (Figure 3A). There were no treatment complications. All discharged patients received an outpatient buprenorphine prescription and 89% received a naloxone overdose kit (Figure 3A). Although 100% of patients were referred to a MAT center, only 45% attended their first appointment and 36% were still engaged at 30 days (Figure 3B). Reasons for loss of engagement included a mix of patient, parent factors, and insurance factors (Figure 3B).
Conclusion(s): ED-based buprenorphine induction is effective for the stabilization of acute opioid withdrawal symptoms in pediatric patients without causing respiratory depression or other complications. Further data is necessary on treatment of patients with severe withdrawal and the barriers adolescents face to OUD engagement after ED discharge.
Figure 1 - Buprenorphine induction algorithm for ED patients presenting with opioid withdrawal
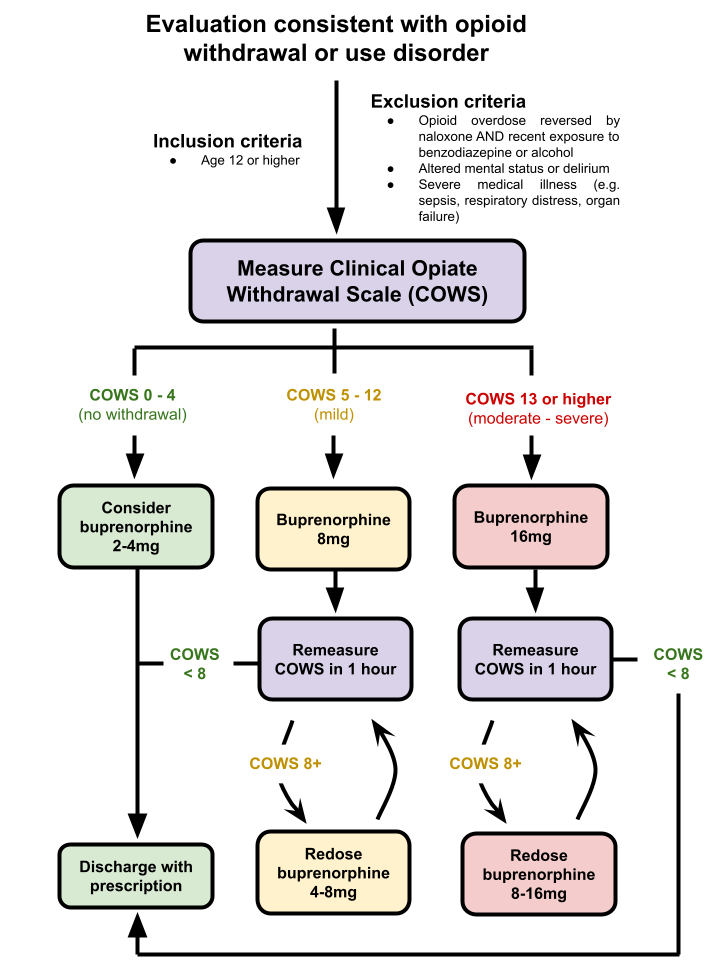 Patients presenting with symptoms of opioid withdrawal are first assessed using the Clinical Opiate Withdrawal Score (COWS) and dosed with an appropriate amount of buprenorphine. Patients are then reassessed with the COWS every hour and given additional doses of buprenorphine as needed until the COWS score decreases to less than 8. The maximum dose of buprenorphine that can be given is 32mg within 24 hours. If the patient’s symptoms are not stable after 2-3 doses of buprenorphine (or the maximum dose is reached), clinicians consider admission to the hospital.
Patients presenting with symptoms of opioid withdrawal are first assessed using the Clinical Opiate Withdrawal Score (COWS) and dosed with an appropriate amount of buprenorphine. Patients are then reassessed with the COWS every hour and given additional doses of buprenorphine as needed until the COWS score decreases to less than 8. The maximum dose of buprenorphine that can be given is 32mg within 24 hours. If the patient’s symptoms are not stable after 2-3 doses of buprenorphine (or the maximum dose is reached), clinicians consider admission to the hospital.Figure 2 - Patient demographic characteristics and co-morbid conditions
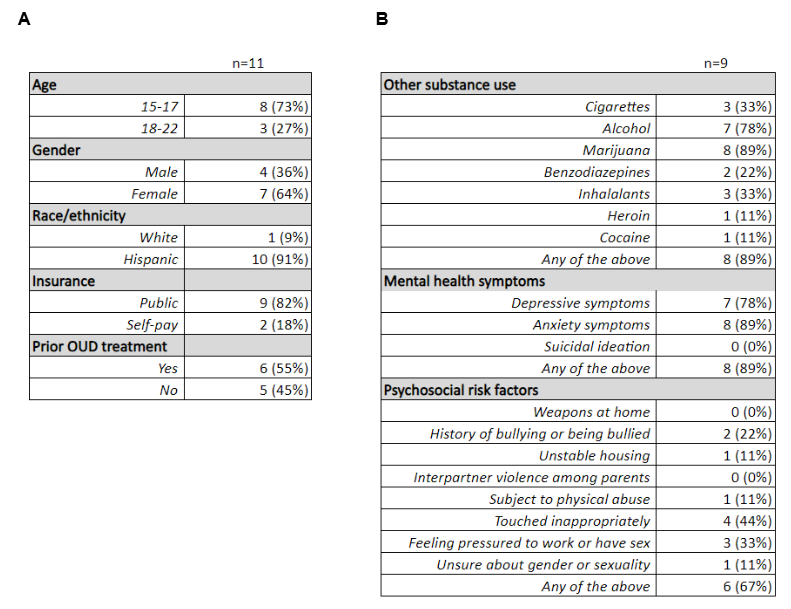 (A) Demographic characteristics of the 11 patients that met inclusion criteria for the study.
(A) Demographic characteristics of the 11 patients that met inclusion criteria for the study. (B) 9/11 patients (82%) completed the ED-DRS and S2BI screening tools. Of those, 89% of patients reported using another substance on the S2BI, of which the most common were marijuana (89%) and alcohol (78%). Patients also reported high rates of comorbid depressive symptoms (78%) and anxiety (89%) on the ED-DRS. 67% reported at least one psychosocial risk factor on the ED-DRS.
Figure 3 - Clinical outcomes after ED-based induction with buprenorphine
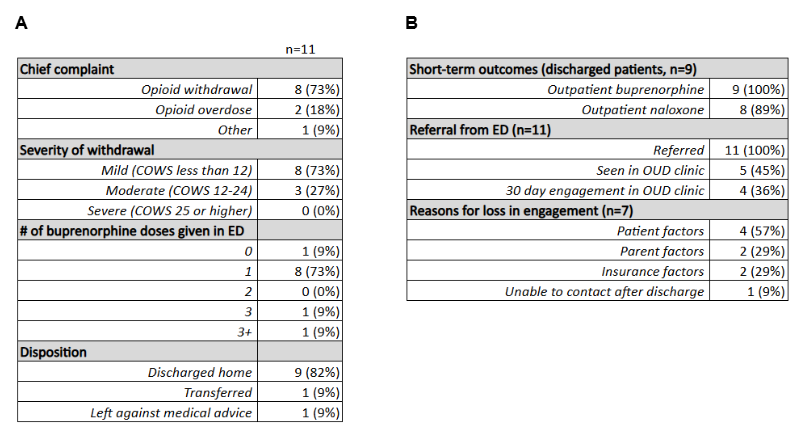 (A) Treatment characteristics among ED patients induced with buprenorphine for opioid withdrawal.
(A) Treatment characteristics among ED patients induced with buprenorphine for opioid withdrawal.(B) Post-discharge outcomes after stabilization with buprenorphine and factors contributing to loss of engagement in treatment.
Figure 1 - Buprenorphine induction algorithm for ED patients presenting with opioid withdrawal
 Patients presenting with symptoms of opioid withdrawal are first assessed using the Clinical Opiate Withdrawal Score (COWS) and dosed with an appropriate amount of buprenorphine. Patients are then reassessed with the COWS every hour and given additional doses of buprenorphine as needed until the COWS score decreases to less than 8. The maximum dose of buprenorphine that can be given is 32mg within 24 hours. If the patient’s symptoms are not stable after 2-3 doses of buprenorphine (or the maximum dose is reached), clinicians consider admission to the hospital.
Patients presenting with symptoms of opioid withdrawal are first assessed using the Clinical Opiate Withdrawal Score (COWS) and dosed with an appropriate amount of buprenorphine. Patients are then reassessed with the COWS every hour and given additional doses of buprenorphine as needed until the COWS score decreases to less than 8. The maximum dose of buprenorphine that can be given is 32mg within 24 hours. If the patient’s symptoms are not stable after 2-3 doses of buprenorphine (or the maximum dose is reached), clinicians consider admission to the hospital.Figure 2 - Patient demographic characteristics and co-morbid conditions
 (A) Demographic characteristics of the 11 patients that met inclusion criteria for the study.
(A) Demographic characteristics of the 11 patients that met inclusion criteria for the study. (B) 9/11 patients (82%) completed the ED-DRS and S2BI screening tools. Of those, 89% of patients reported using another substance on the S2BI, of which the most common were marijuana (89%) and alcohol (78%). Patients also reported high rates of comorbid depressive symptoms (78%) and anxiety (89%) on the ED-DRS. 67% reported at least one psychosocial risk factor on the ED-DRS.
Figure 3 - Clinical outcomes after ED-based induction with buprenorphine
 (A) Treatment characteristics among ED patients induced with buprenorphine for opioid withdrawal.
(A) Treatment characteristics among ED patients induced with buprenorphine for opioid withdrawal.(B) Post-discharge outcomes after stabilization with buprenorphine and factors contributing to loss of engagement in treatment.

