Neonatal Pulmonology - Basic/Translational Science 3
Session: Neonatal Pulmonology - Basic/Translational Science 3
319 - Functional, Histological and Biomolecular Changes Caused by Hyperoxia in the Preterm Rabbit Model of Bronchopulmonary Dysplasia (BPD)
Monday, April 28, 2025
7:00am - 9:15am HST
Publication Number: 319.4808
Chiara Catozzi, Chiesi Farmaceutici, Parma, Emilia-Romagna, Italy; Simone De Meo, University of Siena, Parma, Emilia-Romagna, Italy; Francesca Stretti, Chiesi Farmceutici, Parma, Emilia-Romagna, Italy; Matteo Storti, Chiesi Farmaceutici, Parma, Emilia-Romagna, Italy; Carlotta Boggi, Università di Parma, Parma, Emilia-Romagna, Italy; Chiara Cavallo, Chiesi Farmaceutici S.p.A., Parma, Emilia-Romagna, Italy; Francesca Ravanetti, University of Parma, Parma, Emilia-Romagna, Italy; Luisa Ragionieri, Parma Univeristy - Dept. of Veterinary Sciences, Parma, Emilia-Romagna, Italy; Roberta Ciccimarra, University of Parma, Parma, Emilia-Romagna, Italy; Xabier Murgia Esteve, Consultant, Berango, Pais Vasco, Spain; Francesca Ricci, chiesi farmaceutici S.p.A, PARMA, Emilia-Romagna, Italy; Gino Villetti, Chiesi Farmaceutici, Parma, Emilia-Romagna, Italy; Barbara Bartalesi, University of Siena, siena, Toscana, Italy; Monica Lucattelli, University of Siena, Siena, Toscana, Italy
- XM
Xabier Murgia, Dr. Dr. (he/him/his)
Scientific Consultant
Consultant
Berango, Pais Vasco, Spain
Presenting Author(s)
Background: Bronchopulmonary Dysplasia (BPD) is a multifactorial chronic respiratory disease affecting premature infants. BPD is characterized by perinatal inflammation, impaired lung function and compromised alveolar and vascular development. Postnatal exposure to high oxygen levels represents one of the risk factors involved into the development of BPD.
Objective: This study aimed to investigate the functional, histological and biomolecular changes induced by hyperoxia in the preterm rabbit model of BPD.
Design/Methods: Preterm rabbits were delivered on the 28th day of gestation and exposed to normoxia (21% O2) or hyperoxia (95% O2) until postnatal day 7 (PND7). Term rabbits born on the 31st day of gestation and fostered by their mothers for 4 days were used as age-matched controls. At PND7, lung function (inspiratory capacity and static compliance), morphometric analyses (radial alveolar count [RAC] and acute lung injury [ALI] score) were carried out. The expression of inflammation (IL-1B and CXCL-8) and angiogenesis biomarkers (VEGF-A, ANGPT-1, ANGPT-2, THBS-1) were determined by qRT-PCR. Surfactant protein-C (SFTP-C) was quantified in immunoassayed lung sections with an artificial intelligence-based software (Visiopharm, Denmark) as a marker of alveolar type II (AT II) cells.
Results: Compared with term-born pups, premature birth alone was associated with a slight impairment of lung function, a higher ALI score and a lower number of SFTP-C positive AT II cells. No differences between preterm pups exposed to normoxia and controls were observed in the gene expression of inflammation and angiogenesis biomarkers. The continuous exposure to hyperoxia led to a marked impairment of lung development, denoted by a significant decrease in inspiratory capacity, static compliance, RAC and the number of SFTP-C positive AT II cells. The expression of pro-angiogenic genes (VEGF-A, ANGPT-1) was significantly repressed by hyperoxia, along with the induction of anti-angiogenic genes (THBS-1, ANGPT-2). In preterm pups exposed to hyperoxia, the gene expression of pro-inflammatory mediators (CXCL-8, IL-1B) was significantly higher, in agreement with the higher ALI score.
Conclusion(s): Postnatal exposure to hyperoxia until PND7 impairs the lung development of preterm rabbits. Continuous hyperoxia induces an imbalance of angiogenesis-regulating factors, favoring anti-angiogenic pathways and increasing the expression of genes coding for pro-inflammatory cytokines. Therefore, preterm rabbits exposed to hyperoxia mimic the biomolecular features of human BPD and can be used for anti-inflammatory and pro-angiogenic drug screening studies.
Figure 1
.jpg) Evaluation of lung function, lung morphometry and number of SFTP-C positive cells in premature and term rabbit pups.
Evaluation of lung function, lung morphometry and number of SFTP-C positive cells in premature and term rabbit pups.Lung function measurements (Inspiratory Capacity and Static Compliance) were performed using the Flexivent system; Morphometric analysis (RAC and ALI score); Quantification of SFTP-C positive cells by using Visiopharm software. Results are presented as mean ± SD and analyzed with one-way ANOVA, corrected for multiple comparisons.
* p<0,05; ** p<0,01; *** p<0,001; **** p<0,0001.
Results are presented as mean ± SD and analyzed with one-way ANOVA, corrected for multiple comparisons.
* p<0,05; ** p<0,01; *** p<0,001; **** p<0,0001.
Figure 2
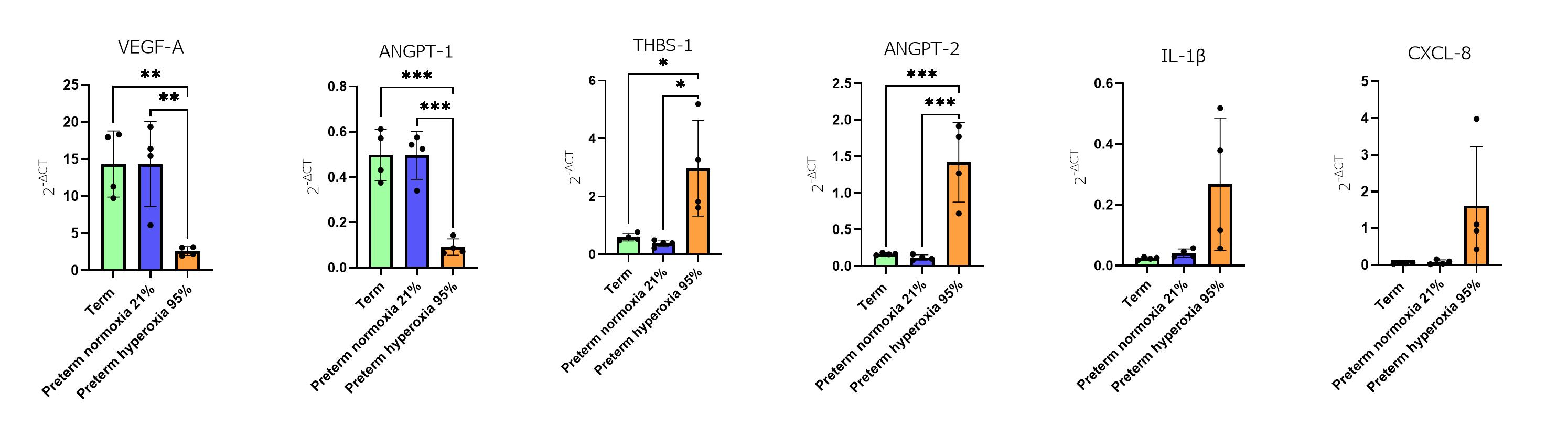 Evaluation of gene expression of inflammation and angiogenesis biomarkers in premature and term rabbit pups.
Evaluation of gene expression of inflammation and angiogenesis biomarkers in premature and term rabbit pups.Gene expression by qRT-PCR (VEGF-A, ANGPT-1, THBS-1, ANGPT-2, IL-1B, CXCL-8).
Results are presented as mean ± SD and analyzed with one-way ANOVA, corrected for multiple comparisons.
* p<0,05; ** p<0,01; *** p<0,001.

