Emergency Medicine 11
Session: Emergency Medicine 11
534 - Variations in Pediatric Sickle Cell Disease (SCD) Clinical Trial Outcomes for Acute Vaso-occlusive Pain Episodes (VOE): Lessons learned from the PECARN STArT Trial
Monday, April 28, 2025
7:00am - 9:15am HST
Publication Number: 534.7068
Claudia R.. Morris, Emory University School of Medicine, Atlanta, GA, United States; Fahd A. Ahmad, Washington University in St. Louis School of Medicine, St. Louis, MO, United States; Gladstone Airewele, Baylor College of Medicine, Houston, TX, United States; Bolanle Akinsola, Emory University School of Medicine, Atlanta, GA, United States; Nitya Bakshi, Yale School of Medicine, New Haven, CT, United States; David Brousseau, Nemours Children's Hospital, Wilmington, DE, United States; Kathleen Brown, Children's National Health System, Washington, DC, United States; Andrew D.. Campbell, Children's National Hospital, Washington, DC, United States; Charles Casper, University of Utah School of Medicine, Salt Lake City, UT, United States; Todd Chang, Children's Hospital Los Angeles, Los Angeles, CA, United States; Corrie E. Chumpitazi, Duke University, Durham, NC, United States; Daniel Cohen, Nationwide Children's Hospital, Bexley, OH, United States; Keli Coleman, Medical College of Wisconsin, Wauwatosa, WI, United States; Andrea T. Cruz, Baylor College of Medicine, Houston, TX, United States; Angela Ellison, Childrens Hospital of Philadelphia, Philadelphia, PA, United States; Melanie Fields, Washington University in St. Louis School of Medicine, St. Louis, MO, United States; Monica Harding, University of Utah, Salt Lake City, UT, United States; Dunia Hatabah, Emory University School of Medicine, Atlanta, GA, United States; Rawan Korman, Emory University School of Medicine, Atlanta, GA, United States; Sara Leibovich, UCSF Benioff Children's Hospital Oakland, Oakland, CA, United States; Chris A. Rees, Emory University School of Medicine, Atlanta, GA, United States; Allison S. Remiker, Medical College of Wisconsin, Milwaukee, WI, United States; Nidhi Singh, Baylor College of Medicine, Houston, TX, United States; Alexis Thompson, Children's Hospital of Philadelphia, Philadelphia, PA, United States; Elliott P.. Vichinsky, UCSF Benioff Children's Hospital Oakland, Oakland, CA, United States; Anthony Villella, Nationwide Childrens Hospital, Columbus, OH, United States; Bridget A. Wynn, Emory University School of Medicine, Atlanta, GA, United States; Carlton Dampier, Emory University School of Medicine, Atlanta, GA, United States

Claudia R. Morris, MD (she/her/hers)
Professor of Pediatrics and Emergency Medicine
Emory University School of Medicine
Atlanta, Georgia, United States
Presenting Author(s)
Background: VOE is the leading cause of emergency department (ED) visits & hospitalizations. FDA-approved drugs for VOE are lacking and all phase-3 randomized controlled trials (RCTs) to date have failed to meet their primary outcomes. The 10-site Pediatric Emergency Care Applied Research Network (PECARN) STArT trial (SCD Treatment with Arginine Therapy) was stopped early for futility to achieve the primary outcome of time-to-crisis-resolution (TCR) after enrolling 271/360 subjects nearly a year ahead of schedule. Given the ongoing shortening of US hospital stays over the last 30 years from >6 days to < 72 hours (hr), TCR is a flawed outcome. TCR variability is influenced by several known demographic factors, but effects of other confounders are unknown.
Objective: To determine independent variables impacting TCR within standard care.
Design/Methods: STArT was 85% powered to detect a ∆TCR of >17hr. Variations in patient characteristics/clinical course were evaluated through 68 variables in subjects randomized to STArT placebo arm. Linear models were used for each variable to explore associations with TCR-hr; univariable & multivariate linear models were run with variables in the latter controlled for age, sex, genotype & HU use chosen a priori as contributing to outcomes; last, a multivariable linear model created by selecting significant (p≤ 0.10) candidate variables in the univariable or adjusted models.
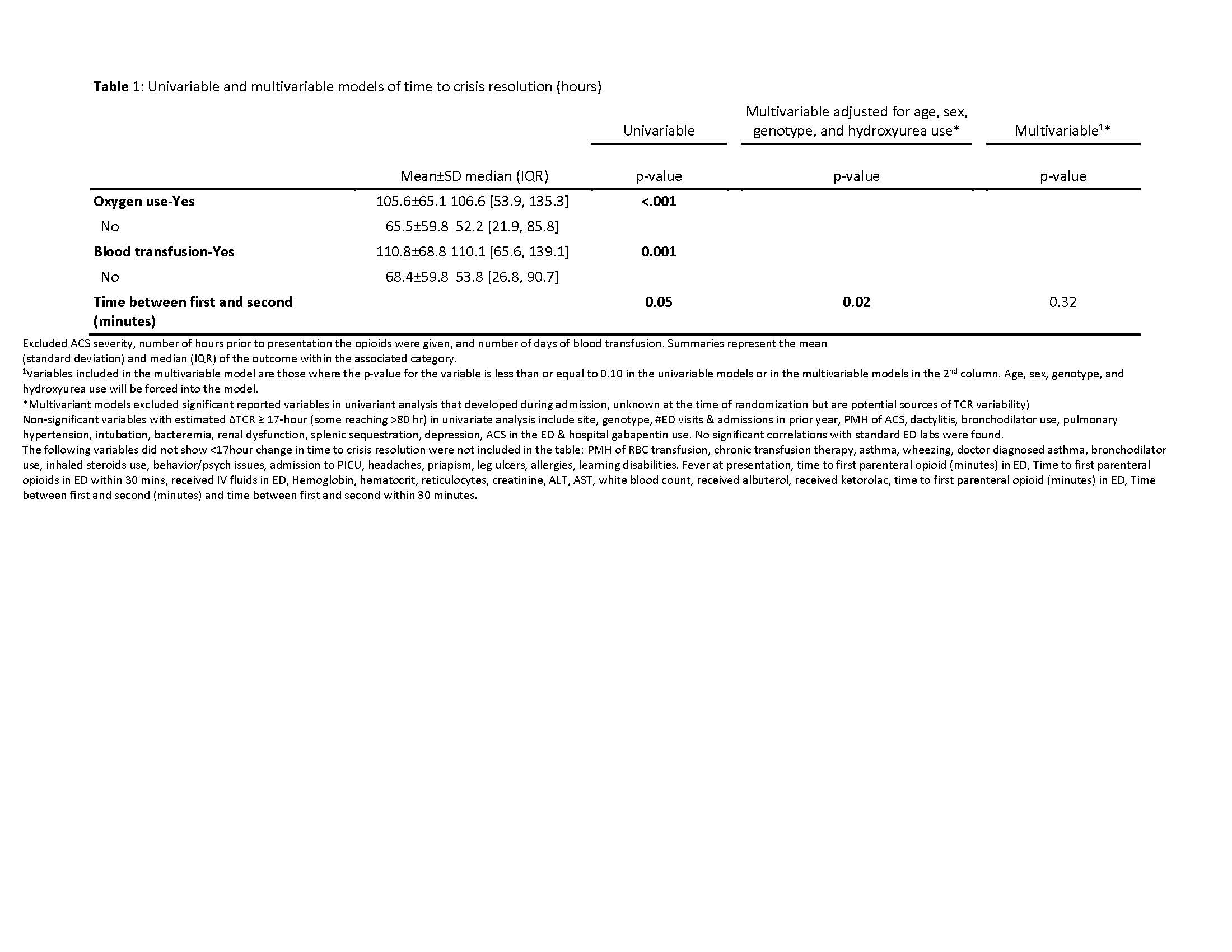
Results: 142 patients were randomized to placebo (mean age 14.2±4.6, 56% male, 69% HbSS/S0, 77% on HU); 1 outlier with a TCR=1724 hr was excluded from analysis. Mean TCR=77±64 hr; median (quartile)=66 (31-110)hr. Table1 highlights significant associations of variables with TCR in univariable/multivariable analysis. Site was the only variable significant in the final linear model (p=0.02). Non-significant variables with estimated ∆TCR≥17-hr (some >80hr) in univariate analysis are also listed in Table1.
Conclusion(s): Standard therapy for SCD is associated with significant TCR variability across sites, influenced by demographics, medical history & clinical course that could confound RCT outcome measures. Larger than expected outliers & SD occurred. Sample size recalculation based on placebo arm data would require randomization of >1500 subjects, highlighting current flaws in SCD-VOE clinical trial design that may prohibit successful drug development. Dialogue between the FDA, researchers & patients is needed to identify more ideal outcome measures beyond TCR, currently FDA-preferred for orphan drug approval. This topic is time-sensitive as other phase-3 RCTs are about to initiate, using potentially suboptimal outcomes
Table 1 Page 1
.jpg)
Table 1 Page 2
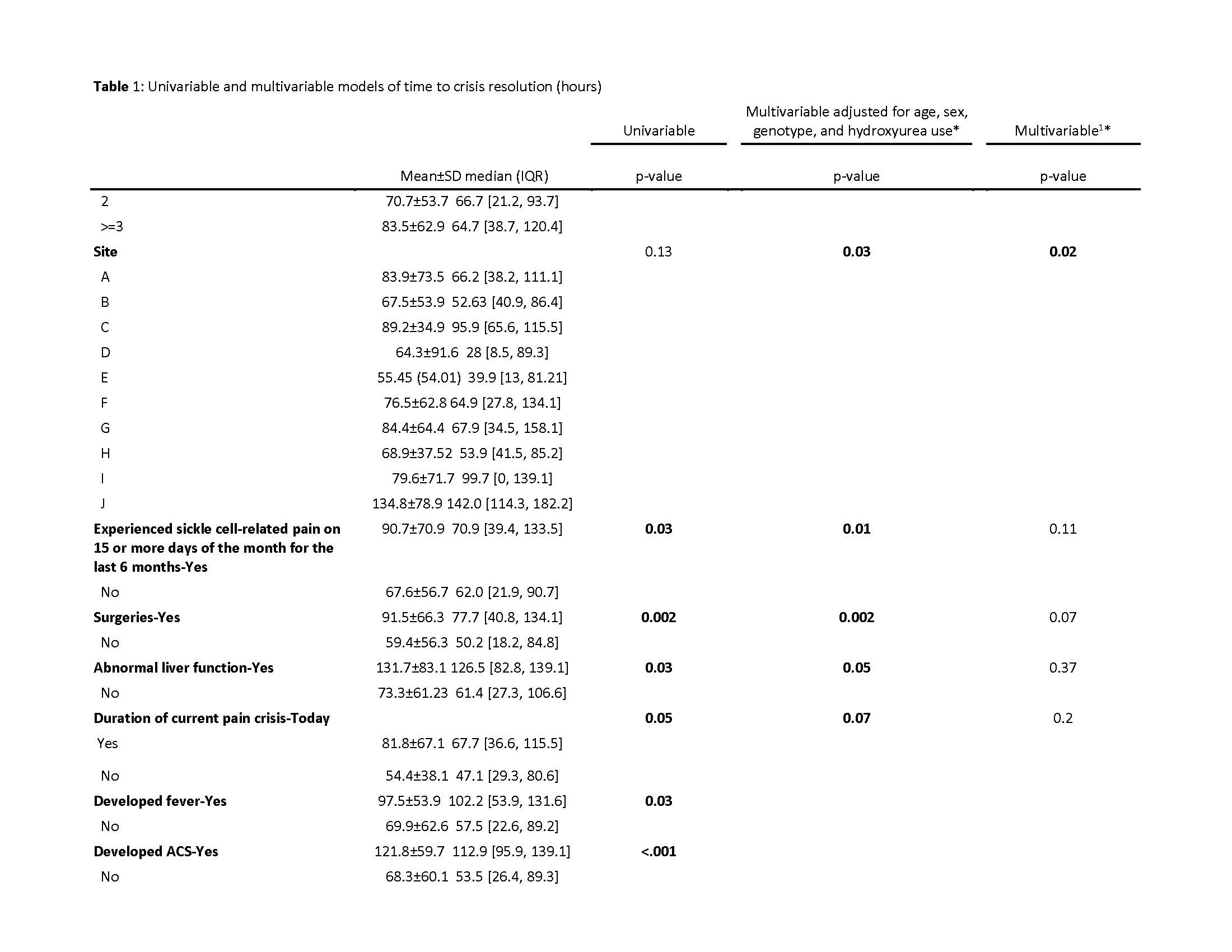
Table 1 Page 3