Neonatal Fetal Nutrition & Metabolism 3
Session: Neonatal Fetal Nutrition & Metabolism 3
634 - Induction of iron deficiency and excess in a human retinal microvascular endothelial cell culture model
Monday, April 28, 2025
7:00am - 9:15am HST
Publication Number: 634.5510
Kaoru Terai, University of Minnesota Medical School, Minneapolis, MN, United States; Timothy R. Monko, University of Minnesota Medical School, Minneapolis, MN, United States; Thomas Bastian, University of Minnesota Medical School, Minneapolis, MN, United States; Ellen C. Ingolfsland, University of Minnesota Medical School, Maple Grove, MN, United States

Ellen C. Ingolfsland, MD
Assistant Professor
University of Minnesota Medical School
Minneapolis, Minnesota, United States
Presenting Author(s)
Background: Derangements in iron homeostasis are common in preterm infants and may impact the development of retinopathy of prematurity (ROP). Our preliminary data from a post-hoc analysis of the Preterm Erythropoietin Neuroprotection Trial identified decreased odds of ROP stage ≥ 2 in infants with evidence of iron deficiency and increased odds in infants with evidence of iron excess (submitted to PAS 2025). We hypothesize that iron deficiency upregulates and iron excess down regulates angiogenesis through the hypoxia-inducible factor 1α-vascular endothelial growth factor (Vegf) pathway.
Objective: To develop and optimize a model of iron deficiency (ID) and iron excess (IE) in human retinal microvascular endothelial cells (HRMECs) to study the impact of ID and IE on retinal angiogenesis
Design/Methods: To induce ID and IE, HRMECs were treated with a range of concentrations of deferoxamine (DFO) or ferric ammonium citrate (FAC): 0, 5, 10, 25, 50 and 100 µM, in 0-2% fetal bovine serum (FBS)-supplemented medium for 24, 48 and 72 hours. Iron status was measured by FerroOrange intensity by microplate reader, transferrin receptor (TFRC) mRNA expression and ferritin protein level. Cell count and survival were measured by count of Hoechst and Propidium Iodide (PI) staining. The effect of ID and IE on angiogenesis was measured by VEGFA mRNA expression by qPCR and prolyl hydroxylase domain 2 (PHD2) protein level by western blot from 10 uM DFO-treated, untreated, and 50 uM FAC-treated HRMECs at 24 and 48h.
Results: 10 uM DFO induced 66% ID by FerroOrange intensity, increased TfRc mRNA expression 2-fold at 24h and reduced ferritin protein to undetectable levels (Fig 1). 50 uM FAC induced 33% IE by FerroOrange, decreased Tfrc mRNA expression 4-fold at 24h and 6-fold at 48h, and increased ferritin protein level 9-fold at 24h and 20-fold at 48h (Fig 1). Results were dependent on percent FBS (Fig 1A, 2A, 2D). Both DFO and FAC-treated cells showed minimal cell death until 72h (Fig 2C,F), but DFO impaired cell proliferation while FAC had minimal effect (Fig 2B,E). DFO-treated HRMECs showed 2-fold and 4-fold increased VegfA mRNA expression at 24 and 48h, respectively (Fig 1B). PHD2 protein was decreased 3-fold in the FAC-treated cells at 24h but not 48h (Fig 2D).
Conclusion(s): At 24 and 48h, DFO induced ID and FAC induced IE in HRMECs grown in 1% FBS supplemented media with low rates of cell death. Though DFO appeared to impair cell proliferation, it upregulated VegfA mRNA expression, while FAC reduced PHD2 levels. These findings support our hypothesis that ID promotes but IE impairs angiogenesis via the HIF-1α pathway.
Figure 1
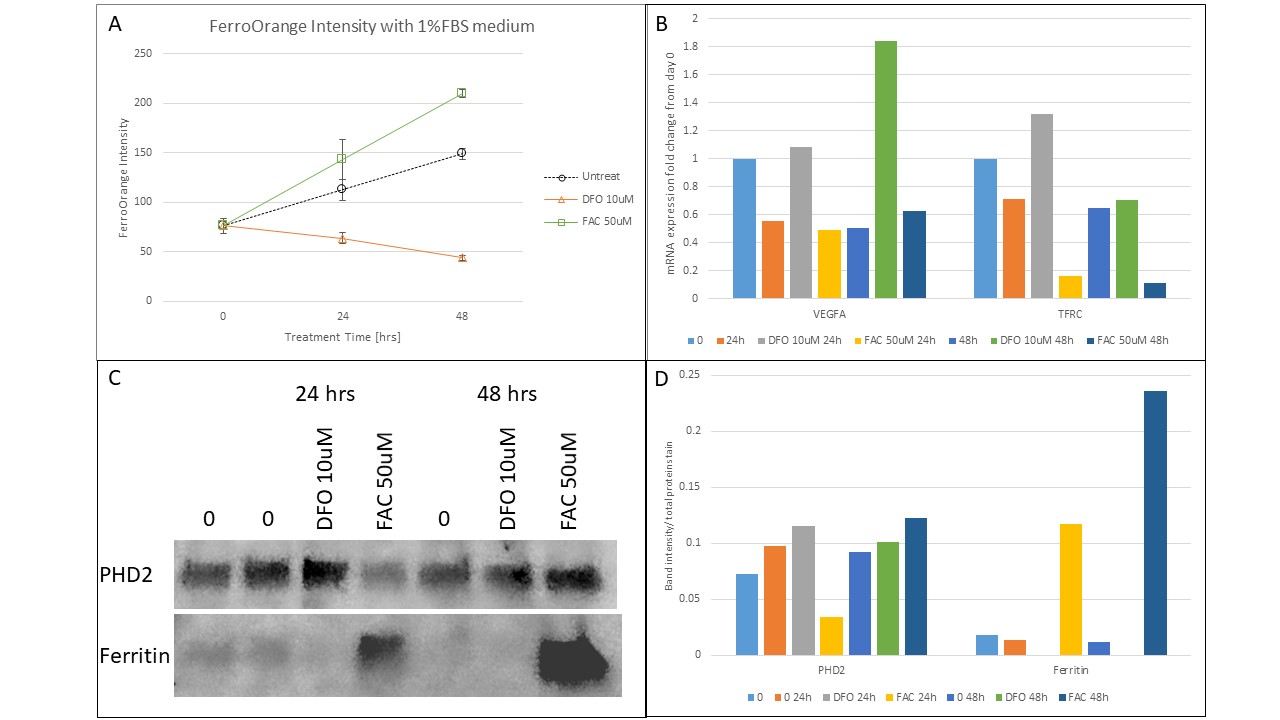 Human retinal microvascular endothelial cells (HRMECs) incubated in 1% fetal bovine serum supplemented growth media with a 10uM DFO or 50 uM FAC. 0, 24, and 48h results of A) FerroOrange intensity, B) mRNA expression of vascular endothelial growth factor-A (Vegfa) and transferrin receptor (TfRc), C-D) protein levels of prolyl hydroxylase domain 2 (PHD2) and ferritin.
Human retinal microvascular endothelial cells (HRMECs) incubated in 1% fetal bovine serum supplemented growth media with a 10uM DFO or 50 uM FAC. 0, 24, and 48h results of A) FerroOrange intensity, B) mRNA expression of vascular endothelial growth factor-A (Vegfa) and transferrin receptor (TfRc), C-D) protein levels of prolyl hydroxylase domain 2 (PHD2) and ferritin.Figure 2
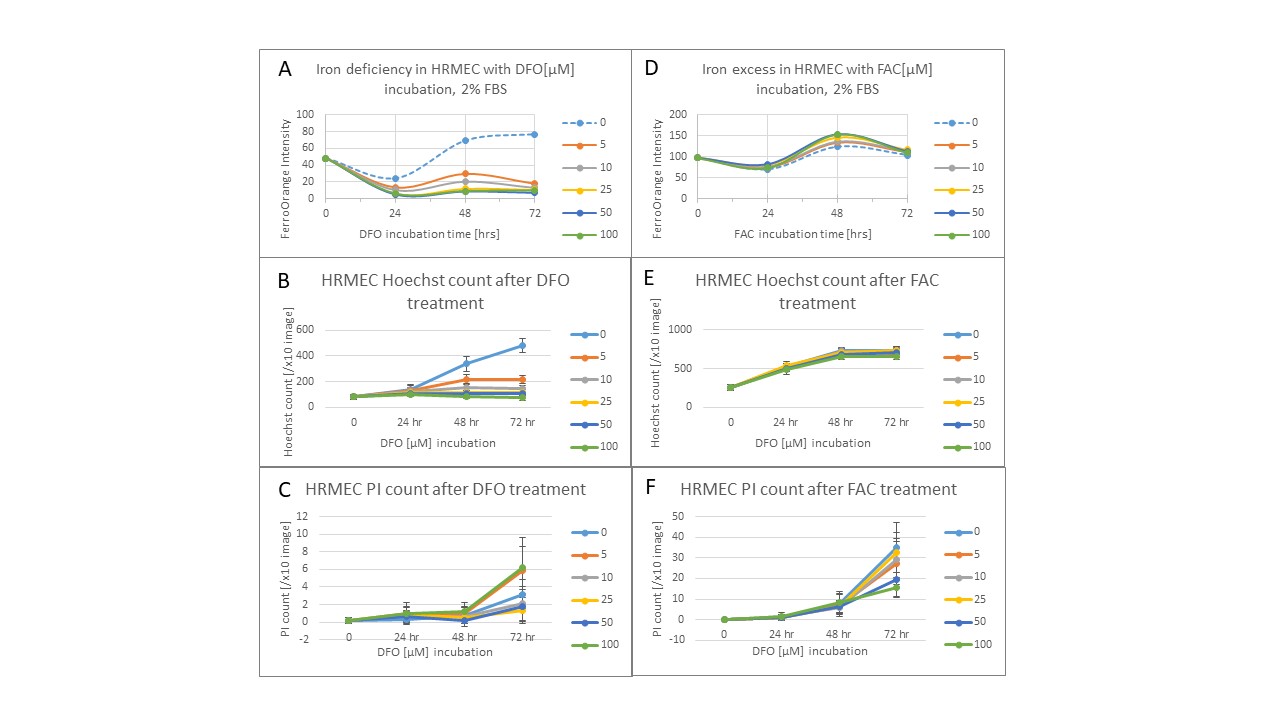 Human retinal microvascular endothelial cells (HRMECs) incubated in 2% fetal bovine serum supplemented growth media with a range of concentrations of deferoxamine (DFO) to induce iron deficiency or ferric ammonia citrate (FAC) to induce iron excess. 0, 24, 48, and 72h results of FerroOrange intensity, Hoechst-stained cell count, propidium iodide (PI)-stained cell count of DFO or FAC treated cells, respectively (A-C, D-F).
Human retinal microvascular endothelial cells (HRMECs) incubated in 2% fetal bovine serum supplemented growth media with a range of concentrations of deferoxamine (DFO) to induce iron deficiency or ferric ammonia citrate (FAC) to induce iron excess. 0, 24, 48, and 72h results of FerroOrange intensity, Hoechst-stained cell count, propidium iodide (PI)-stained cell count of DFO or FAC treated cells, respectively (A-C, D-F). 
