Neonatal GI Physiology & NEC 4
Session: Neonatal GI Physiology & NEC 4
700 - A Digestive Cartridge Improves Survival and Reduces Intestinal Injury in a Murine Model of Necrotizing Enterocolitis
Monday, April 28, 2025
7:00am - 9:15am HST
Publication Number: 700.3678
Sarah Z. Wang, Boston Children's Hospital, Boston, MA, United States; Djanira Fernandes, Boston Children's Hospital, Boston, MA, United States; Savas Tsikis, Boston Children's Hospital, Boston, MA, United States; Thomas Hirsch, Beth Israel Deaconess Medical Center, Boston, MA, United States; Scott Fligor, Boston Children's Hospital, Boston, MA, United States; Mikayla Quigley, Boston Children's Hospital, Boston, MA, United States; Amy Pan, Boston Children's Hospital, Boston, MA, United States; Greta Loring, Alcresta Therapeutics, WALTHAM, MA, United States; Stephen J. Davia, Alcresta Therapeutics, Inc., Brighton, MA, United States; Mark Puder, Boston Children's Hospital, Boston, MA, United States
- SW
Sarah Z. Wang, MD, MPH (she/her/hers)
Research Fellow
Boston Children's Hospital
Boston, Massachusetts, United States
Presenting Author(s)
Background: Necrotizing enterocolitis (NEC) is a life-threatening gastrointestinal illness affecting premature infants. Formula feeding is a major risk factor for NEC. The composition of formula, including fat content, impacts NEC severity. Premature, formula-fed neonates are lipase-deficient, leading to impaired fat digestion. The Immobilized Lipase Cartridge (ILC) is an FDA-approved device that connects in-line with enteral feeding systems. The ILC hydrolyzes fat into more readily absorbable free fatty acid forms prior to reaching the patient, and is FDA-approved for enteral nutrition support in patients age 2 years and older with fat malabsorption. We hypothesized that use of a new, smaller ILC prototype designed specifically for neonatal enteral feeding will reduce mortality and disease severity in a murine model of NEC.
Objective: To evaluate the effect of an ILC-digested enteral formula on NEC mortality and severity.
Design/Methods: NEC was induced in C57BL/6J mouse pups via oral gavage of formula containing E. coli lipopolysaccharide (2.5 ug/g) and hypoxia exposure (10 min twice daily at 5% O2/95% N2) from post-natal day (P) 4 through 6. Littermates were randomized to one of three treatment groups to reduce litter-specific effects (Fig 1): dam-fed (controls), NEC + undigested formula (placebo cartridge), and NEC + ILC-digested formula. The primary outcome was survival to P7. Two masked observers assessed clinical sickness and macroscopic gut appearance on P7. Terminal ileum histology was evaluated. Statistical analysis was performed using analysis of variance (ANOVA) or unpaired t-test where applicable, with values expressed as the mean and standard error of the mean (SEM).
Results: Breastmilk controls had lower mortality than NEC groups (P=0.0019) (Fig 2). Mice administered ILC-digested formula had lower mortality (50.0% v 75.0%, P=0.003), lower clinical sickness scores (4.25 v 8.33, P=0.006), and improved macroscopic gut scores (2.55 v 4.83, P=0.0001) compared to mice administered undigested formula. On representative histology, the NEC + ILC group had improved intestinal villus architecture compared to the NEC + placebo group (Fig 3).
Conclusion(s): Administration of enteral formula pre-digested by an Immobilized Lipase Cartridge improved survival compared to undigested formula in a murine model of NEC. ILC use significantly improved clinical sickness and macroscopic gut appearance scores, and histologic severity of NEC in the terminal ileum. The current findings support further investigation of the ILC as a non-invasive preventative therapy for NEC in humans.
Figure 1
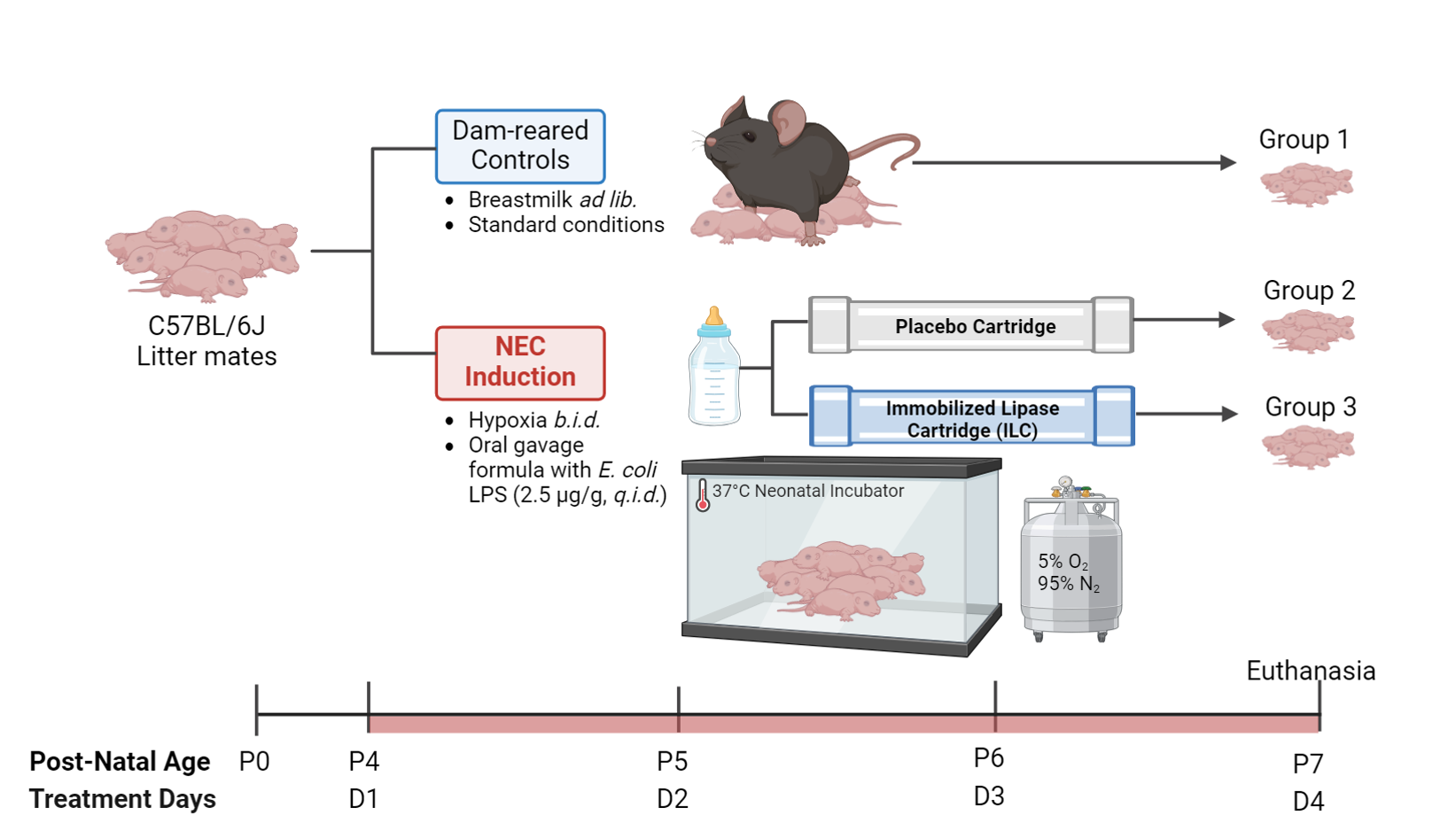 Figure 1. Experimental design investigating the effects of enteral formula pre-digested by an Immobilized Lipase Cartridge in a necrotizing enterocolitis mouse model. Group 1 are breastmilk-fed controls. Groups 2 and 3 undergo experimental induction of NEC via a combination of oral gavage feeding with formula containing E. coli-derived lipopolysaccharide (2.5 ug/g) and twice-daily hypoxia exposure from post-natal day 4 through 6 as described previously (Nolan et al., STAR Protoc, 2021). Group 2 receives formula processed through an empty, placebo cartridge. Group 3 receives formula pre-digested by the ILC. Animals are sacrificed on P7. NEC – necrotizing enterocolitis; ILC – Immobilized Lipase Cartridge; ad lib – ad libitum (liberally); q.i.d. – four times per day.
Figure 1. Experimental design investigating the effects of enteral formula pre-digested by an Immobilized Lipase Cartridge in a necrotizing enterocolitis mouse model. Group 1 are breastmilk-fed controls. Groups 2 and 3 undergo experimental induction of NEC via a combination of oral gavage feeding with formula containing E. coli-derived lipopolysaccharide (2.5 ug/g) and twice-daily hypoxia exposure from post-natal day 4 through 6 as described previously (Nolan et al., STAR Protoc, 2021). Group 2 receives formula processed through an empty, placebo cartridge. Group 3 receives formula pre-digested by the ILC. Animals are sacrificed on P7. NEC – necrotizing enterocolitis; ILC – Immobilized Lipase Cartridge; ad lib – ad libitum (liberally); q.i.d. – four times per day.Figure 2
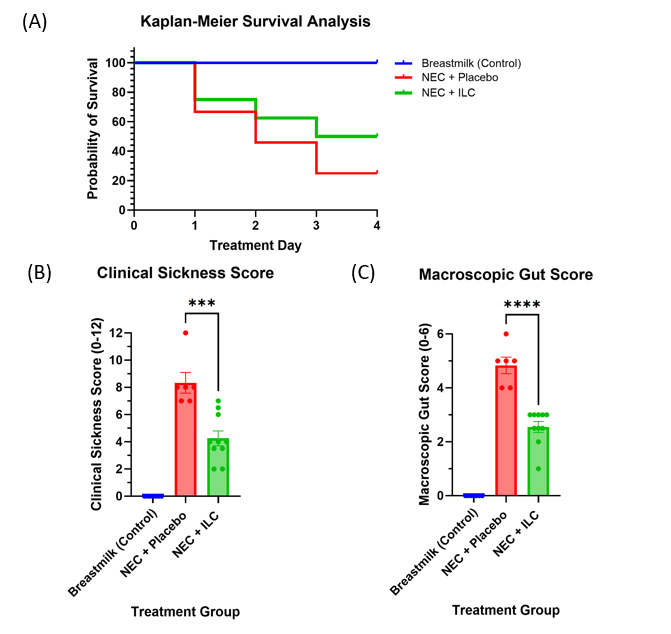 Figure 2. Survival and clinical outcomes in a necrotizing enterocolitis mouse model. (A) Survival analysis; (B) Clinical sickness score; (C) Macroscopic gut score. Two independent masked observers assessed clinical sickness and macroscopic gut scores on day of euthanasia according to a validated NEC severity scoring system (Zani et al., Eur J Pediatr Surg, 2008). Values are expressed as the mean +/- standard error of the mean (SEM). NEC – necrotizing enterocolitis; ILC – Immobilized Lipase Cartridge.
Figure 2. Survival and clinical outcomes in a necrotizing enterocolitis mouse model. (A) Survival analysis; (B) Clinical sickness score; (C) Macroscopic gut score. Two independent masked observers assessed clinical sickness and macroscopic gut scores on day of euthanasia according to a validated NEC severity scoring system (Zani et al., Eur J Pediatr Surg, 2008). Values are expressed as the mean +/- standard error of the mean (SEM). NEC – necrotizing enterocolitis; ILC – Immobilized Lipase Cartridge.Figure 3
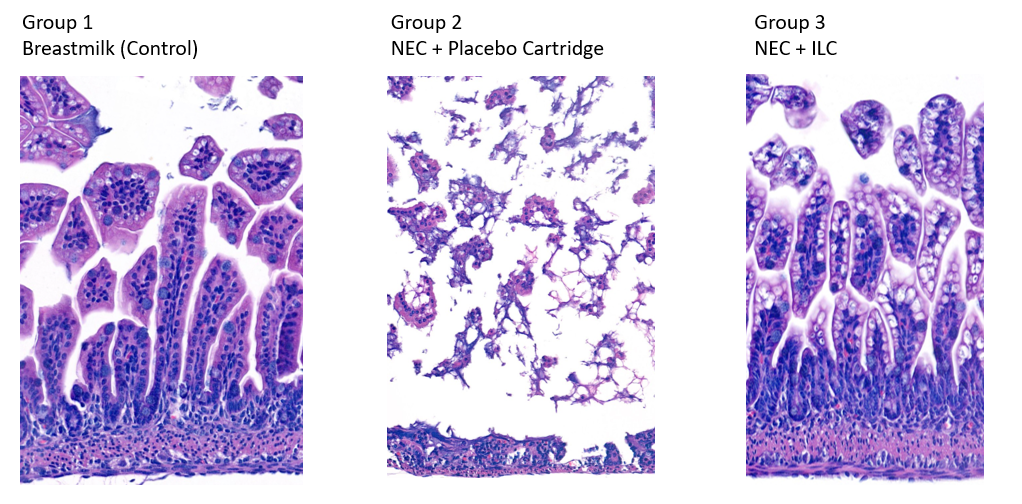 Figure 3. Representative H&E-stained terminal ileum specimens in a necrotizing enterocolitis mouse model. Group 1 – breastmilk-fed neonatal mice had normal villus architecture. Group 2 – NEC mice fed formula processed through a placebo cartridge had villus sloughing and disrupted basement membrane. Group 3 – NEC mice fed formula processed through the ILC had preserved villus architecture. 10X magnification. NEC – necrotizing enterocolitis; ILC – Immobilized Lipase Cartridge.
Figure 3. Representative H&E-stained terminal ileum specimens in a necrotizing enterocolitis mouse model. Group 1 – breastmilk-fed neonatal mice had normal villus architecture. Group 2 – NEC mice fed formula processed through a placebo cartridge had villus sloughing and disrupted basement membrane. Group 3 – NEC mice fed formula processed through the ILC had preserved villus architecture. 10X magnification. NEC – necrotizing enterocolitis; ILC – Immobilized Lipase Cartridge.
