Neonatal/Infant Resuscitation 3
Session: Neonatal/Infant Resuscitation 3
382 - Fetal-Maternal Oxygen Transfer During Physiological-Based Cord Clamping in Preterm Lambs
Monday, April 28, 2025
7:00am - 9:15am HST
Publication Number: 382.4825
Ebony R. Cannata, The Ritchie Centre, Hudson Institute of Medical Research, Noble Park, Victoria, Australia; Kelly J. Crossley, The Ritchie Centre, Clayton, Victoria, Australia; Alison Thiel, Hudson Institute of Medical Research, clayton, Victoria, Australia; Cailin Diedericks, Monash University, St Helena, Victoria, Australia; Paige J. Riddington, Monash University, Clayton, Victoria, Australia; Ilias Nitsos, Hudson Institute, Clayton, Victoria, Australia; Indya M. Davies, Hudson Institute of Medical Research, Clayton, Victoria, Australia; Janneke Dekker, Leiden University Medical Center, Leiden, Zuid-Holland, Netherlands; Graeme Polglase, Monash University, Melbourne, Victoria, Australia; Douglas Blank, Dept of Paediatrics, Monash University and Monash Newborn, Port Melbourne, Victoria, Australia; Stuart B. Hooper, Monash University, Black Rock, Victoria, Australia

Ebony R. Cannata, BSc (hons) (she/her/hers)
PhD Candidate
The Ritchie Centre, Hudson Institute of Medical Research
Leongatha, Victoria, Australia
Presenting Author(s)
Background: Physiological-based cord clamping (PBCC) involves ventilating infants while attached to the umbilical cord and avoids the loss in cardiac output associated with cord clamping. The dynamics of placental oxygen transfer during PBCC are unknown as the infant can continue to receive oxygen from the mother while also commencing pulmonary oxygen exchange. We hypothesised that maternal-fetal oxygen transfer will continue during PBCC but will be reduced by pulmonary ventilation and increased oxygenation.
Objective: We aimed to investigate the effects of pulmonary ventilation during PBCC on maternal-fetal oxygen transfer.
Design/Methods: Pregnant ewes and their fetuses (n=8; 127d of gestation; term 147d) were instrumented with catheters and flow probes under general anaesthesia. Ewes were mechanically ventilated with a 0.3 fraction of inspired oxygenation (FiO2). Before umbilical cord clamping, lambs were intubated and mechanically ventilated, increasing the FiO2 every 10 minutes from 0.21, 0.5, then 1.0. Uteroplacental oxygen uptake was calculated by taking paired blood gas samples from the maternal artery and uterine vein, with uterine artery blood flow. Fetal oxygen uptake was calculated by taking paired blood gas samples from the umbilical vein and fetal femoral artery, with common umbilical vein blood flow. A power analysis determined that 6-8 animals were required to test differences in the partial pressure of oxygen (PO2) levels between groups. Data were analysed using a mixed-effects analysis with a Holm-Šídák post-hoc test.
Results: The PO2 in both the maternal artery and uterine vein increased after ventilating lambs with increasing FiO2 (p=0.0054, p< 0.0001; Figure 1A-B), but uterine artery blood flow and uteroplacental oxygen uptake remained relatively constant (p=0.5084, p=0.4622; Figure 1C-D). PO2 levels in the fetal umbilical vein and femoral artery increased with increasing FiO2 (p=0.0321, p=0.0102; Figure 2A-B). Umbilical venous blood flow and oxygen uptake by the lamb across the placenta were both significantly reduced with increasing FiO2 over time (p=0.0018, p< 0.0001; Figure 2C-D).
Conclusion(s): During PBCC, when the lambs were ventilated with high FiO2 levels, oxygen transfer across the placenta appeared to reverse and pass from lamb to mother. This is indicated by higher PO2 levels in the femoral artery versus umbilical vein, negative fetal oxygen uptake values and increasing PO2 levels in the uterine vein, which increased maternal arterial PO2 levels. Thus, PBCC may protect newborns against hyperoxia during the onset of pulmonary ventilation.
Figure 1. Maternal oxygen levels during PBCC with increasing oxygen in the lamb.
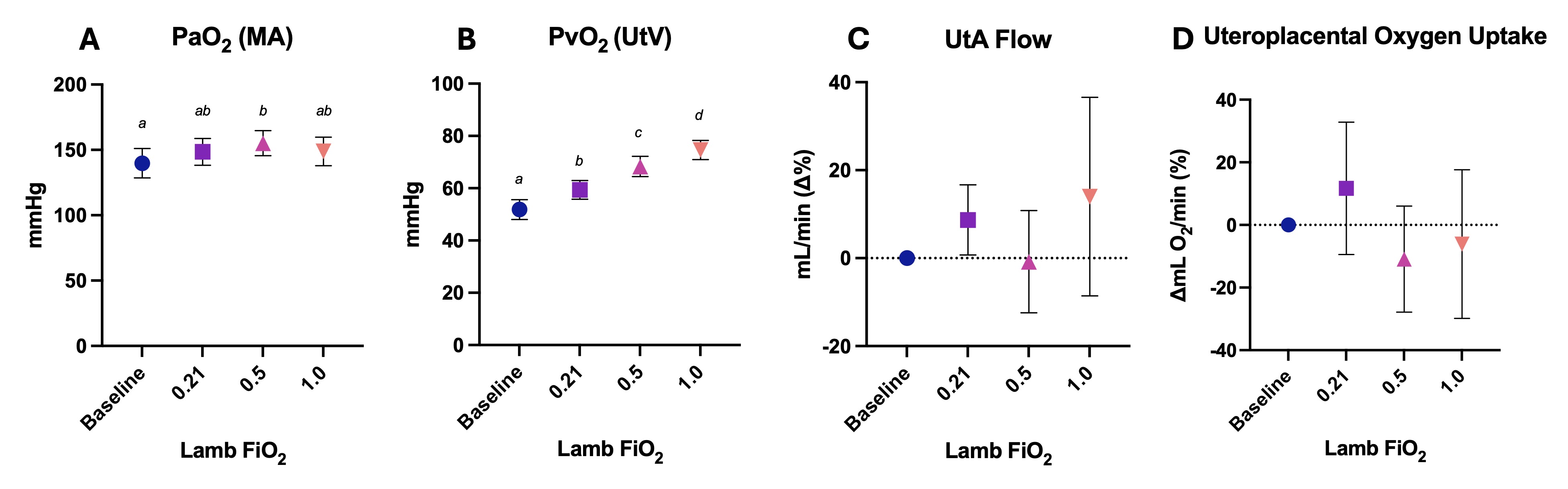 A) Partial pressure of oxygen (PaO2) in maternal arterial (MA) blood, B) partial pressure of oxygen (PvO2) in uterine venous (UtV) blood, C) uterine artery (UtA) blood flow, and D) and uteroplacental oxygen uptake during ventilation of lambs with different fraction of inspired oxygen (FiO2) levels. Uteroplacental oxygen uptake was calculated by multiplying the difference between MA and UtV oxygen content by UtA blood flow. Data are presented as mean ± SEM. Values that do not share a common letter are significantly different from each other (p < 0.05).
A) Partial pressure of oxygen (PaO2) in maternal arterial (MA) blood, B) partial pressure of oxygen (PvO2) in uterine venous (UtV) blood, C) uterine artery (UtA) blood flow, and D) and uteroplacental oxygen uptake during ventilation of lambs with different fraction of inspired oxygen (FiO2) levels. Uteroplacental oxygen uptake was calculated by multiplying the difference between MA and UtV oxygen content by UtA blood flow. Data are presented as mean ± SEM. Values that do not share a common letter are significantly different from each other (p < 0.05).Figure 2. Lamb oxygen levels during PBCC with increasing oxygen levels.
.jpg) A) Partial pressure of oxygen (PvO2) in umbilical venous (UmbV) blood, B) partial pressure of oxygen (PaO2) in femoral arterial (FA) blood, C) UmbV blood flow, and D) and fetal oxygen uptake during ventilation of lambs with different fraction of inspired oxygen (FiO2) levels. Fetal oxygen uptake was calculated by multiplying the difference between UmbV and FA oxygen content, by UmbV blood flow. Data are presented as mean ± SEM. Values that do not share a common letter are significantly different from each other (p < 0.05).
A) Partial pressure of oxygen (PvO2) in umbilical venous (UmbV) blood, B) partial pressure of oxygen (PaO2) in femoral arterial (FA) blood, C) UmbV blood flow, and D) and fetal oxygen uptake during ventilation of lambs with different fraction of inspired oxygen (FiO2) levels. Fetal oxygen uptake was calculated by multiplying the difference between UmbV and FA oxygen content, by UmbV blood flow. Data are presented as mean ± SEM. Values that do not share a common letter are significantly different from each other (p < 0.05).
