Neonatal Follow-Up 3: Long-Term Outcomes in NICU Graduates
Session: Neonatal Follow-Up 3: Long-Term Outcomes in NICU Graduates
589 - Poorer Neonatal Neurobehavioral Regulation Is Associated With Longitudinal Epigenetic Age Deceleration in Children That Were Born Very Preterm
Monday, April 28, 2025
7:00am - 9:15am HST
Publication Number: 589.6400
Priyadarshni Patel, Emory University, Atlanta, GA, United States; Marie Camerota, The Warren Alpert Medical School of Brown University, Providence, RI, United States; Brian S.. Carter, Children's Mercy Hospitals and Clinics, Kansas City, MO, United States; Jennifer Check, Wake Forest School of Medicine of Wake Forest Baptist Medical Center, Winston-Salem, NC, United States; Jennifer helderman, WFB1-AtriumWH, Clemmons, NC, United States; Julie Hofheimer, University of North Carolina at Chapel Hill, Durham, NC, United States; Elisabeth McGowan, Women & Infants Hospital of Rhode Island, Providence, RI, United States; Charles R. Neal, University of Hawaii John A Burns School of Medicine, Kailua, HI, United States; Thomas Michael O'Shea, University of North Carolina at Chapel H, Chapel Hill, NC, United States; Steven L. Pastyrnak, Michigan State University College of Human Medicine, Grand Rapids, MI, United States; Carmen J. Marsit, Emory University Rollins School of Public Health, Atlanta, GA, United States; Barry Lester, The Warren Alpert Medical School of Brown University, Providence, RI, United States; Todd Everson, Emory University, Atlanta, GA, United States

Priyadarshni Patel, PhD
Post Doctoral Fellow
Emory University
Atlanta, Georgia, United States
Presenting Author(s)
Background: Children born very preterm face heightened risks for developmental delays. The Neonatal
Intensive Care Unit Network Neurobehavioral Scale (NNNS) assesses neurobehavioral profiles
in infants and has been linked to later developmental outcomes. Epigenetic age acceleration is
a marker of biological aging that has been associated with chronic disease in adults, while in
children it is unclear if age acceleration is a marker of negative or positive health. The
relationship between NNNS profiles and epigenetic age acceleration during childhood is
understudied.
Objective: This study aimed to investigate associations between NNNS neurobehavioral profiles and
epigenetic age acceleration at 3, 4, and 5 years in children born very preterm participating in the
Neonatal Neurobehavior and Outcomes in Very Preterm Infants (NOVI) study.
Design/Methods: Participants included 540 children born < 30 weeks gestation with NNNS and DNA methylation
data. Epigenetic age was estimated using the Pediatric buccal (PedBE) epigenetic clock. Age
acceleration was calculated by regressing epigenetic age on chronological age at each
timepoint. Previously validated NNNS profiles characterized six distinct groups based on
neurobehavioral patterns at NICU discharge; profiles 5 and 6 demonstrated the most poorly
regulated neurobehavior (hypo- and hyper-arousal, respectively) and profile 1, the most typical
functioning, was treated as the reference. Cross sectional and linear mixed-effects models
examined associations between each NNNS profile and PedBE epigenetic age acceleration at
each timepoint and longitudinally. Additional analyses of individual NNNS summary scores
identified specific domain associations.
Results: Cross-sectional results demonstrated significantly lower age acceleration at ages 3 (profile 6:
β=-0.42, p=0.019) and 4 years (profile 5: β=-0.28, p=0.039). Longitudinal trends indicated
significantly lower age acceleration across ages for profiles 5 (p=0.029) and 6 (p=0.081).
Analyses of individual NNNS summary scores highlighted that higher stress-abstinence was
significantly associated with epigenetic age deceleration (β=-1.05, p=0.029).
Conclusion(s): Our results indicated that elevated neurobehavioral dysregulation risk, and specifically stress
abstinence, assessed near NICU discharge were linked to reduced epigenetic age acceleration
in childhood. Further research should examine trajectories of neurobehavioral regulation, stress,
and epigenetic aging to better understand the dynamic associations of behavioral and biological
processes in children born very preterm.
Patterns of NNNS z-scores across individual assessments for all six profiles among infants; Profile 6 represents the atypical profile (black) and Profile 1 represents the optimal profile (green).
.jpg)
Longitudinal association between NNNS profiles and epigenetic age.
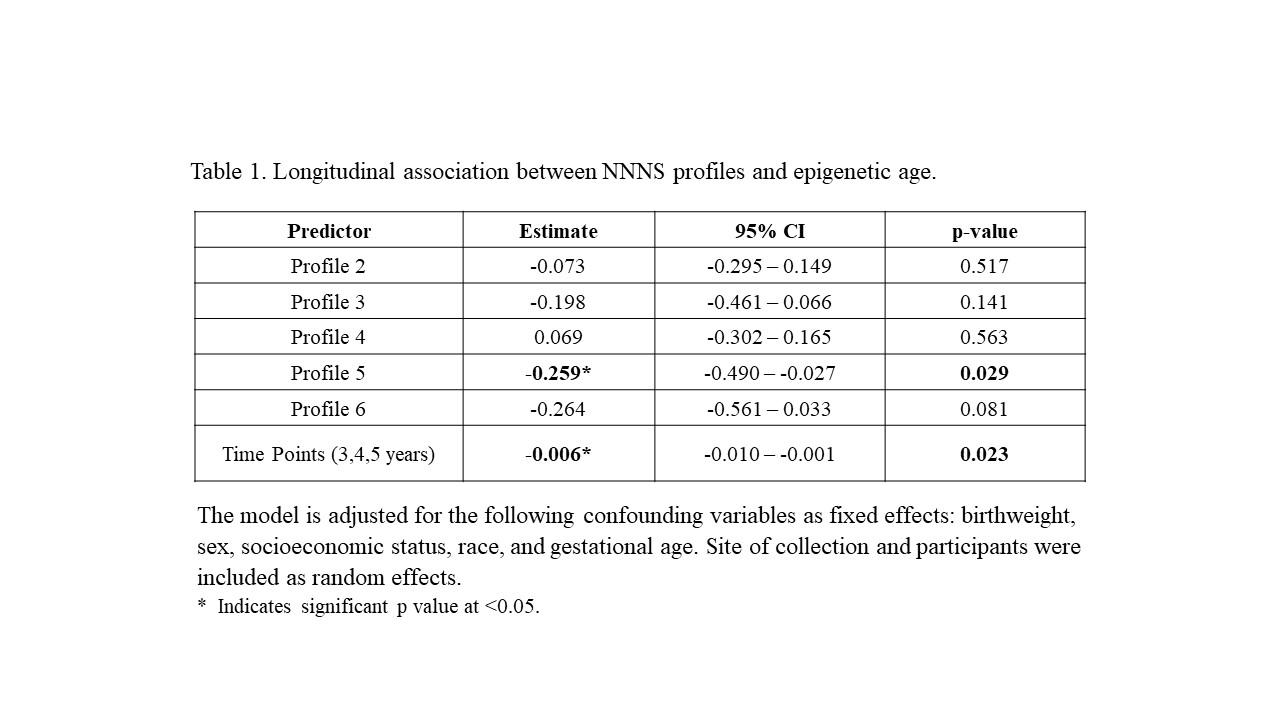 The model is adjusted for the following confounding variables as fixed effects: birthweight, sex, socioeconomic status, race, and gestational age. Site of collection and participants were included as random effects.
The model is adjusted for the following confounding variables as fixed effects: birthweight, sex, socioeconomic status, race, and gestational age. Site of collection and participants were included as random effects.
