Critical Care 4
Session: Critical Care 4
502 - Hypoglycemia as a Complication of Citrate Anticoagulation in Continuous Renal Replacement Therapy: Mechanism and Clinical Implications
Monday, April 28, 2025
7:00am - 9:15am HST
Publication Number: 502.5521
Constance E. Poplos, University of Tennessee Health Science Center College of Medicine, Memphis, TN, United States; Kathleen Zani, University of Arkansas for Medical Sciences College of Medicine, Little Rock, AR, United States; Stephanie A. Storgion, UTHSC LeBonheur, Memphis, TN, United States; Becky M. Landman, Le Bonheur Children's Hospital, Hernando, MS, United States; Alexandra L. Schaller, UTHSC, LeBonheur Children's Hospital, Memphis, TN, United States

Constance E. Poplos, MS (she/her/hers)
student
University of Tennessee Health Science Center College of Medicine
Memphis, Tennessee, United States
Presenting Author(s)
Background: The use of continuous renal replacement therapy (CRRT) requires careful choice of anticoagulation. Calcium citrate is favorable due to its effective clot prevention with only regional effects. In neonates weighing < 10 kg, citrate anticoagulation, administered in a solution containing 2.45% dextrose, is frequently used. Due to the high rates needed relative to patient size for effective anticoagulation, discontinuation of CRRT, and therefore citrate, can lead to rapid changes in glucose infusion rates (GIR). Repeated and prolonged episodes of hypoglycemia put patients at risk for unfavorable neurological outcomes including seizures, permanent neurodevelopmental deficits, and death. Hypoglycemia incidence and outcomes during calcium citrate anticoagulation are largely unexplored in the literature. Here, we aim to characterize hypoglycemia related to citrate anticoagulation and implement a protocolized management approach to limit hypoglycemia in patients with calcium citrate anticoagulation on CRRT.
Objective: The objective of this study is to determine if critical hypoglycemic events (glucose < 70) occurred in patients less than < 10kg receiving calcium citrate anticoagulation therapy.
Design/Methods: A retrospective review was conducted on patients from 2017 to 2023 that were less than 10kg and using calcium citrate anticoagulation on CRRT, independent of any other extracorporeal device. Hypoglycemic events were then collected and correlated with sudden or expected discontinuation of CRRT within 4 hours prior to hypoglycemic event. The independent glucose infusion rate during calcium citrate anticoagulation was then calculated prior to these events.
Results: Ten patients weighing < 10 kg were on CRRT with citrate anticoagulation from 2017-2023. Thirty hypoglycemic events occurred in these patients with 21 events (70%) occurring within 4 hours after both planned and unplanned termination of CRRT. The GIR provided by the calcium citrate anticoagulation prior to these events ranged from 2.1 to 11.3. Hypoglycemic events ranged from 38 mg/dL to 70 mg/dL. No patients had seizure activity, but mental status changes were noted. Only one patient had a glucose check within one hour of discontinuation of CRRT.
Conclusion(s): These findings highlight the need for monitoring of sudden drops in GIR to avoid potentially disastrous and irreversible complications. With this data, we implemented a standardized protocol for glucose monitoring and replacement after discontinuation of citrate anticoagulation.
Image 1
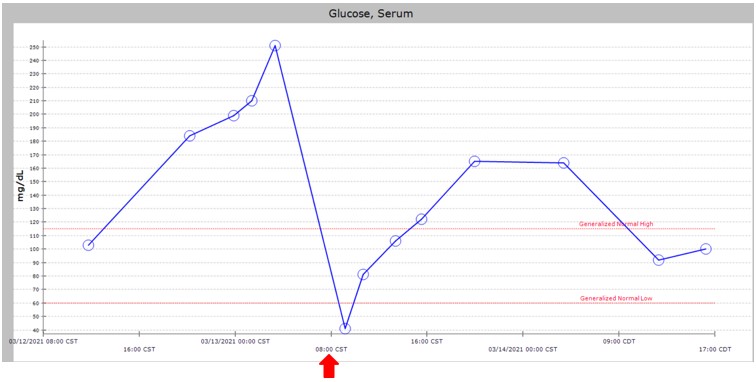 Example of Hypoglycemic Event: Patient weight 2.5 kg, Citrate Rate 75 ml/hr citrate. Provided GIR 12 mg/kg/min with abrupt cessation at 800am (marked with red arrow).
Example of Hypoglycemic Event: Patient weight 2.5 kg, Citrate Rate 75 ml/hr citrate. Provided GIR 12 mg/kg/min with abrupt cessation at 800am (marked with red arrow).Table 1
table 1.pdfInformation on demographics, indication for initiation of CRRT, glucose infusion rate, and number of hypoglycemic events directly correlating with discontinuation of CRRT with citrate anticoagulation.

