Genomics/Epigenomics 1
Session: Genomics/Epigenomics 1
383 - Rapid whole genome sequencing in the NICU: A process time comparison
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 383.6214
Tanner Ellsworth, University of Utah, Salt Lake City, UT, United States; Chelsea Solorzano, University of Utah School of Medicine, Salt Lake City, UT, United States; Paul Estabrooks, University of Utah School of Medicine, Salt Lake City, UT, United States; Martin Tristani-Firouzi, University of Utah School of Medicine, Salt Lake City, UT, United States; Sabrina Malone Jenkins, University of Utah, Salt Lake City, UT, United States

Tanner Ellsworth, MD, MPH (he/him/his)
Neonatal-Perinatal Medicine Fellow
University of Utah
Salt Lake City, Utah, United States
Presenting Author(s)
Background: Rapid whole genome sequencing (rWGS) is a valuable tool in diagnosis and treatment planning for rare genetic disorders the NICU. As rWGS enters standard neonatology practice, there remain many barriers to equitable utilization including time constraints, subspecialty access, and standard practice patterns across systems with different resources. Without careful review, there is a potential for inequitable distribution of this lifesaving tool based on geographic region and NICU levels.
Objective: This study compares rWGS patterns and process times between Level III (L3) and IV (L4) NICU settings to identify differences that may inform a streamlined process for more equitable implementation and utilization of rWGS.
Design/Methods: This is a retrospective review of neonates admitted to a Level III or level IV NICU within a single health system who underwent rWGS. Time stamps were noted for various steps in the process such as admission, genetic consultation/note, rWGS consent/order, blood draw, and sample receipt at lab. Data are reported as number with percentage and median with IQR. Statistical analysis was performed using Fisher’s Exact and Mann-Whitney U tests.
Results: 73 neonates were identified who received rWGS in a L3 (n=8) or L4 setting (n=65). Significantly fewer received genetic consultation at L3 compared to L4 (Table 1, 63% v 92%, p=0.04). At L3 level, 80% of genetic consultations occurred after rWGS order had already been placed, compared to 15% at L4. All underwent consent process prior to blood draw; however, consent was not documented in EMR for 25% of the infants at L3 level. All infants at L4 level had formal consent in the EMR.
Between the two settings, the interval from admission to rWGS order trended toward significance with a L4 median time of 4.2 days compared to 9.1 days at L3 (p=0.055). The interval from order to blood draw was significantly shorter at L4 (3.5 v. 12.0 hours, p=0.043). There was no significant difference between time of blood draw to receipt of sample at the laboratory (31 vs. 43 hours, p=0.073).
Conclusion(s): Significant differences in rWGS processing between L3 and L4 settings indicate inequities for patients, which presents opportunity for improvement in the process—specifically with earlier involvement of genetic specialists and formalized consent. The L4 setting demonstrated more consistent involvement of genetic specialists, documentation of consent, and quicker identification of neonates who would undergo rWGS. All settings may benefit from a standardized rWGS process workflow to ensure this tool is utilized to its full potential regardless of setting.
Table 1
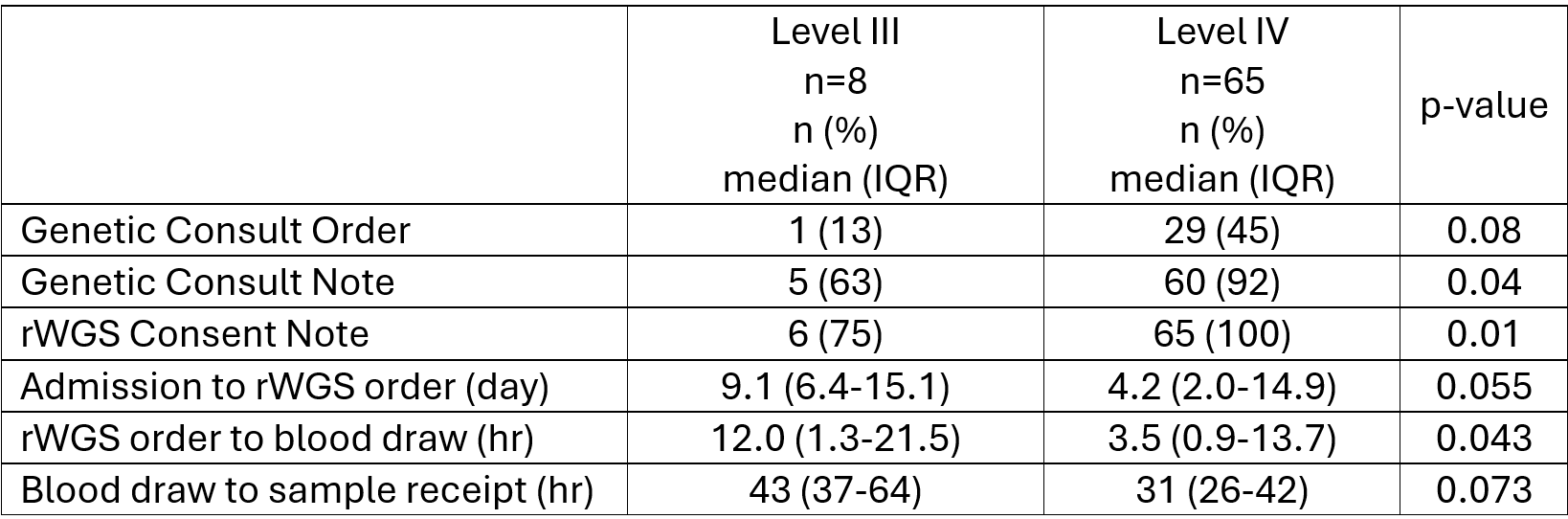 Comparison of rWGS processing in Level III vs. Level IV settings
Comparison of rWGS processing in Level III vs. Level IV settings
