General Pediatrics 1
Session: General Pediatrics 1
704 - U.S. FDA Approval of Pediatric Artificial Intelligence and Machine Learning-Enabled Medical Devices
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 704.6616
Ryan Brewster, Ariadne Labs, Brigham and Women's Hospital, Boston, MA, United States; Susmitha Wunnava, Harvard Medical School, Boston, MA, United States; Florence Bourgeois, Boston Children's Hospital, Boston, MA, United States

Ryan Brewster, MD
Resident Physician
Ariadne Labs, Brigham and Women's Hospital
Boston, Massachusetts, United States
Presenting Author(s)
Background: Artificial intelligence- and machine learning (AI/ML)-enabled medical devices are a rapidly growing product type that require specific validation in children to ensure safe and appropriate use in the pediatric population. However, the U.S. Food and Drug Administration (FDA) does that device labels provide information on whether children were included in the development and testing of algorithms used in these devices.
Objective: To quantify the availability of AI/ML-enabled devices authorized for children and assess reporting of algorithm validation in the pediatric populations.
Design/Methods: All AI/ML-enabled medical devices marketed in the US through March 2024 were identified from the FDA’s website and classified as authorized for use in children ( < 18 years) or adults only. The description of clinical performance testing in product summary documentation was manually evaluated to determine whether information was provided on the age of patients in the validation dataset and if so, whether pediatric patients were included.
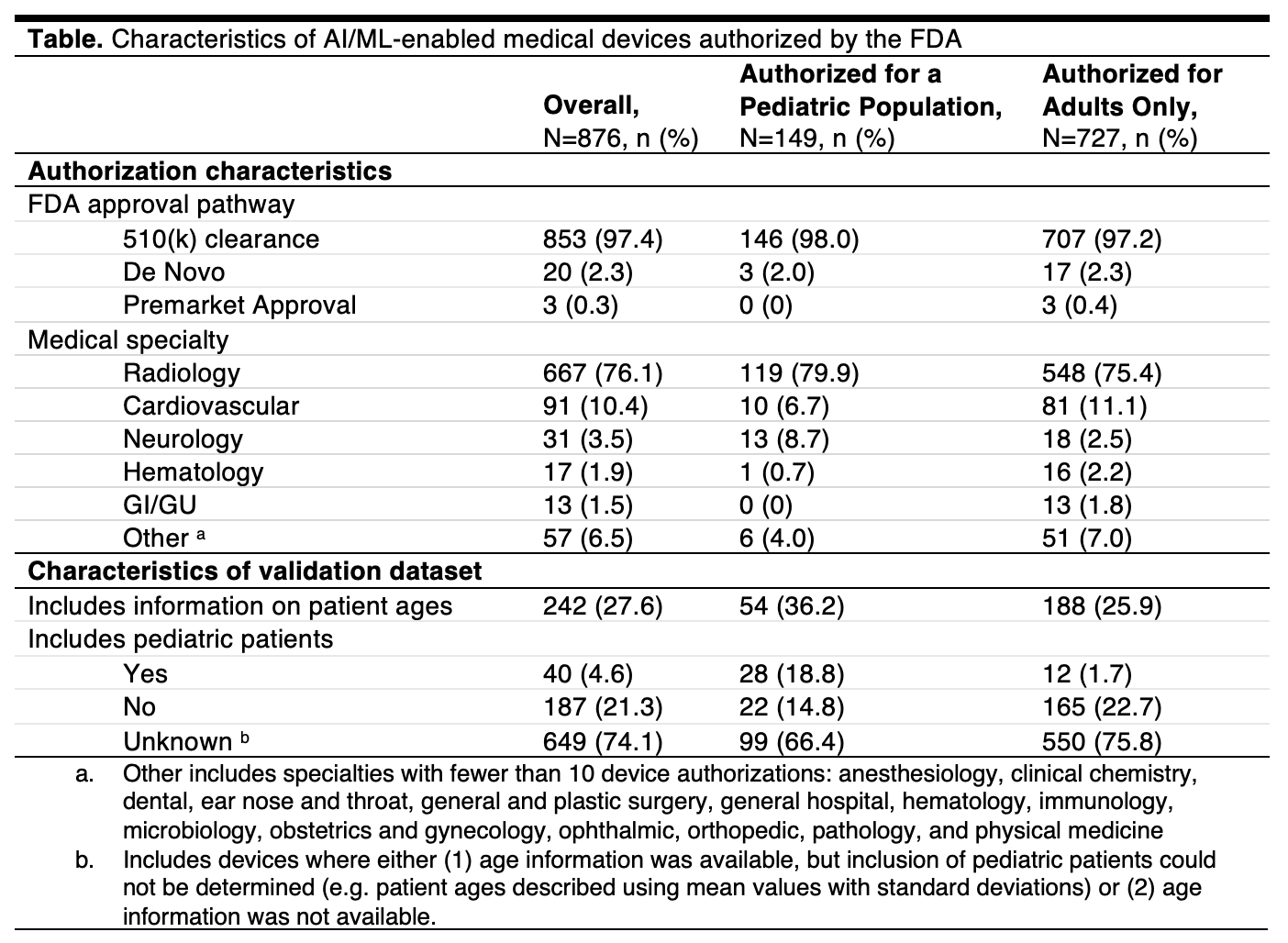
Results: A total of 876 AI/ML-enabled medical devices were analyzed, most of which were authorized after 2021 (62.7%) through the 510(k) clearance pathway (97.4%) (Figure, Table). The most common medical specialties were radiology (76.1%), cardiovascular (10.4%), and neurology (3.5%). Age information for patients in the validation datasets was reported for 242 (27.6%) devices. There were 149 AI/ML-enabled medical devices labeled for pediatric use, corresponding to 17.0% of all AI/ML-enabled device authorizations. For 36.2% (n=54) of pediatric devices, approval summaries described the ages of patients in the validation datasets. Overall, 18.8% (n=28) of pediatric AI/ML-devices reported validation datasets that included children, while 14.8% (n=22) were validated using adult data only. The remaining 66.4% (n=99) did not report whether pediatric patients were studied.
Conclusion(s): Despite rapid growth of AI/ML-enabled devices, only a small number have been authorized for pediatric use. Among these, only few disclosed information in labeling on whether algorithm validation was performed in pediatric cohorts and even fewer described datasets that included children. The current regulatory framework may expose children to differential performance of algorithms and potential safety risks. To ensure safe, equitable, and effective development and implementation, pediatric AI/ML-enabled devices should be validated using representative datasets with complete documentation on pediatric testing and authorization.
Characteristics of AI/ML-enabled medical devices authorized by the FDA
Trends in annual FDA authorization of AI/ML-enabled medical devices
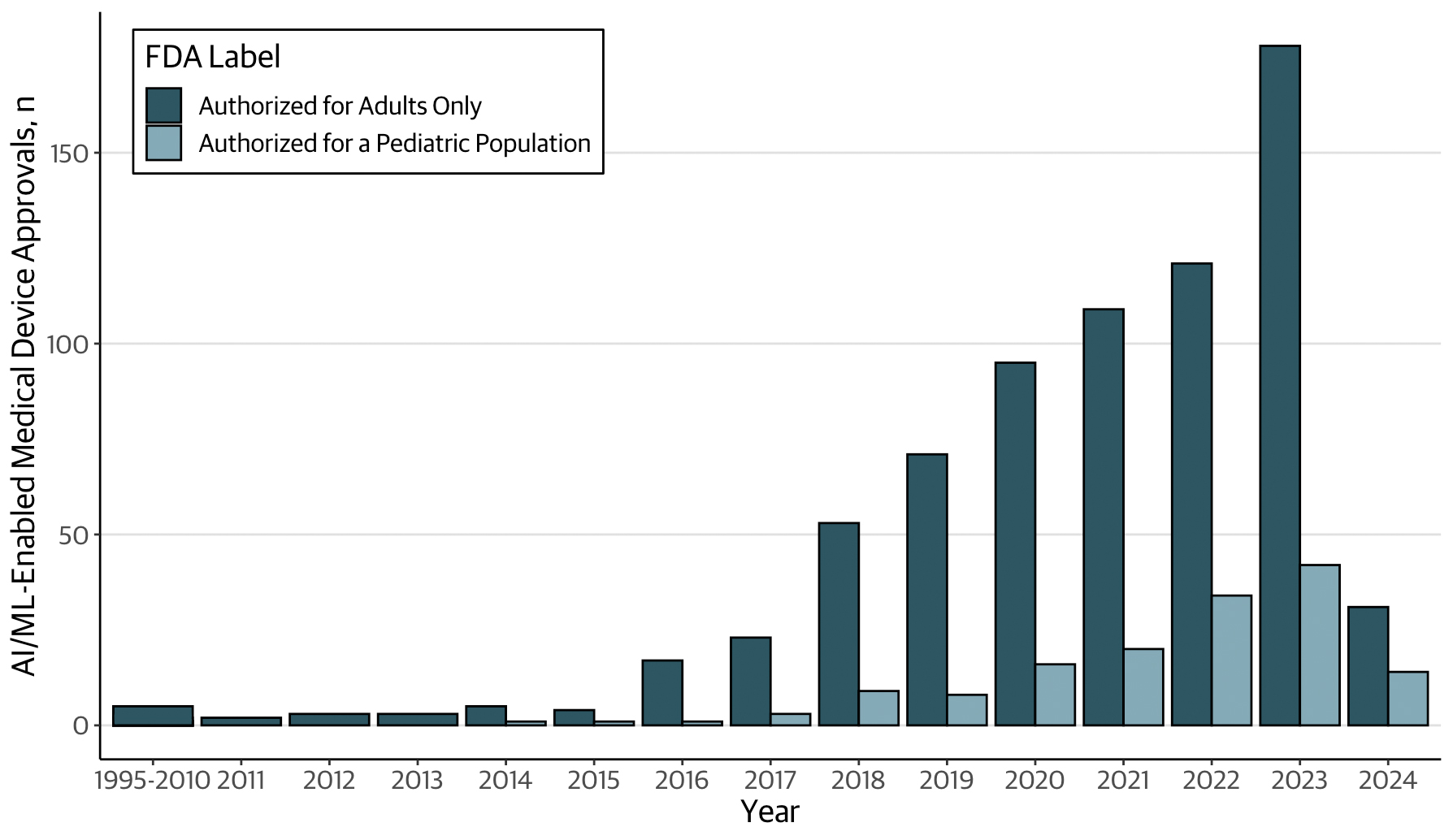
Characteristics of AI/ML-enabled medical devices authorized by the FDA

Trends in annual FDA authorization of AI/ML-enabled medical devices


