Immunizations/Delivery 2
Session: Immunizations/Delivery 2
688 - Neonatal Vaccination: A Ten-Year Retrospective Study in a Large Delivery Center
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 688.5786
Maria Laura Dona Mourao, Beth Israel Deaconess Medical Center, Boston, MA, United States; Adrian Baca-Arzaga, Holtz Children's Hospital Jackson Memorial Hospital, Miami, FL, United States; Marianna Castellanos, The Children's Hospital at Montefiore, Bronx, NY, United States; Rhys Johnstone, Beth Israel Deaconess Medical Center, Boston, MA, United States; David Miedema, Beth Israel Deaconess Medical ctr, Beverly, MA, United States; Al Ozonoff, Harvard Medical School, Boston, MA, United States; Oludare A. Odumade, University of Minnesota Medical School, Minneapolis, MN, United States; Asimenia Angelidou, Harvard Medical School, Boston, MA, United States

Maria Laura Dona Mourao, MD
Post Doc Research Fellow
Beth Israel Deaconess Medical Center
Boston, Massachusetts, United States
Presenting Author(s)
Background: Newborns are at increased risk of preventable morbidity and mortality. The American Academy of Pediatrics (AAP) recommends administering the first dose of hepatitis B vaccine (HBV) between 0-30 days of life (as of 2017, within 24 hours of life if birth weight (BW)≥2000g, and the earlier of 30 days of life or discharge if BW < 2000g), followed by the first dose of the pneumococcal vaccine (PCV) at two months of age among other vaccines. However, adherence to these guidelines is often suboptimal, resulting in incomplete or delayed vaccination.
Objective: We sought to a) analyze rates and timeliness of HBV vaccination in the Neonatal Intensive Care Unit (NICU) vs. Mother-Baby Unit (MBU), b) compare HBV and PCV vaccination rates between preterm and term infants in the NICU, and c) determine associations of vaccination status with clinical and sociodemographic factors.
Design/Methods: This is a retrospective, single-center study in Massachusetts examining HBV and PCV vaccination rates and timeliness for infants admitted between 2013-2023. Based on AAP recommendations, vaccination status was categorized as (1) per AAP guidelines, (2) delayed, and (3) incomplete. Since AAP recommendations differ by BW, we employed time-to-event analysis stratified by BW to assess vaccination delays in the NICU. The influence of clinical (BW, preterm status), sociodemographic factors (race, ethnicity, primary language) and the COVID-19 pandemic on the vaccination status of NICU infants was determined by multinomial logistic regression.
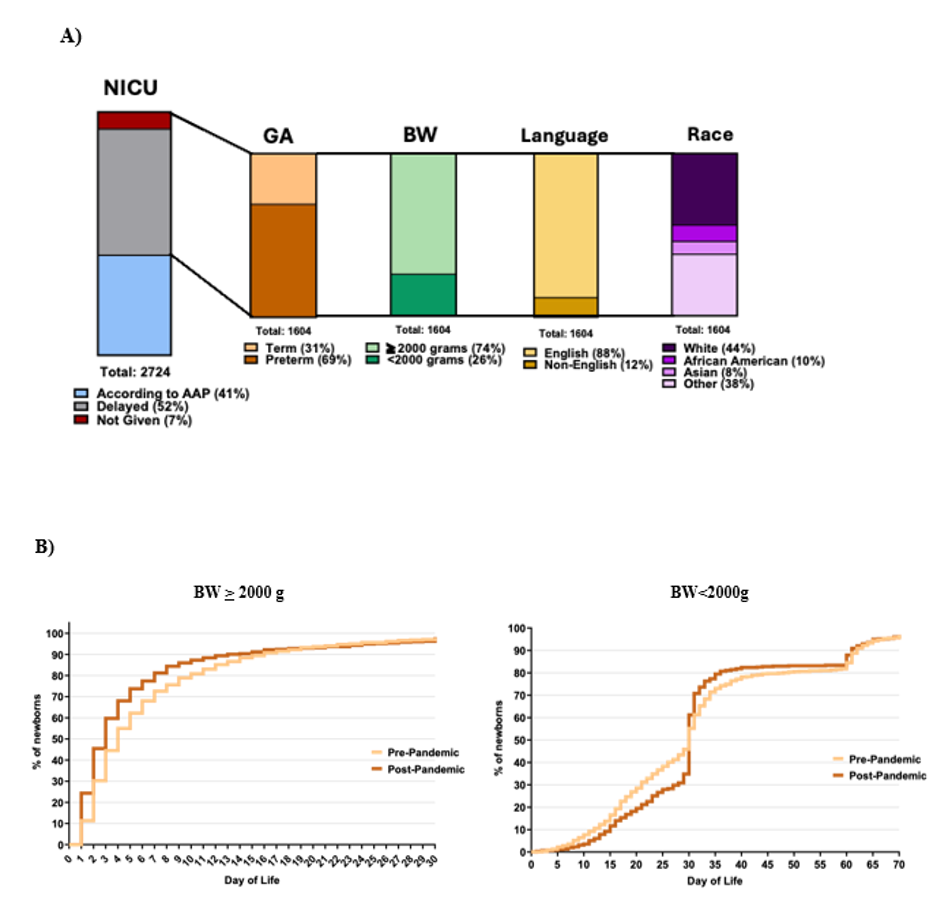
Results: Among 49,867 infants admitted to the MBU and 4,935 infants admitted to the NICU over a 10-year period, HBV vaccination rates peaked at 94.8% and at 95.3% in 2019 and 2020, respectively. HBV vaccination rates declined in both units during the COVID-19 pandemic, to 92.4% for MBU and 90.9% for the NICU in 2023 (Fig.1). Fifty nine percent of infants admitted to the NICU experienced delayed or incomplete birth HBV vaccination (Fig.2): factors associated with HBV vaccination delay included pre-pandemic administration, prematurity, birth weight ≥ 2000g, and Black/African American race (Table 1). Hispanic patients were more likely to be discharged with incomplete PCV vaccination compared to non-Hispanic patients.
Conclusion(s): Preterm status was a key factor in delayed HBV vaccination. Post-pandemic, delayed vaccination rates dropped, possibly reflecting greater vaccine awareness among parents and providers. Perceptions and attitudes toward early-life vaccination, including potential racial biases, should be examined as contributors to differing vaccine practices.
Rates of HBV vaccination from 2013-2023 in the MBU (blue line) compared to the NICU (red line) pre and post-pandemic with black line indicating the start of the COVID-19 pandemic
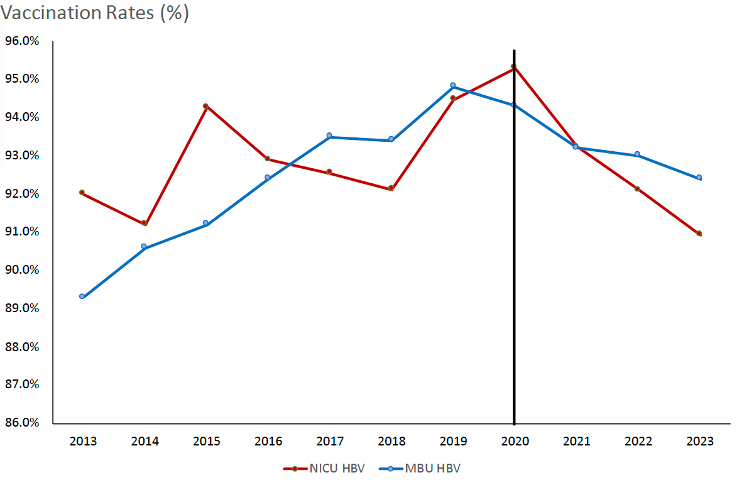
Table 1: Multinomial logistic regression of factors associated with delayed or incomplete HBV vaccination, based on 2017 AAP guidance, among infants born at BIDMC and admitted to the NICU between 2018 and 2023.
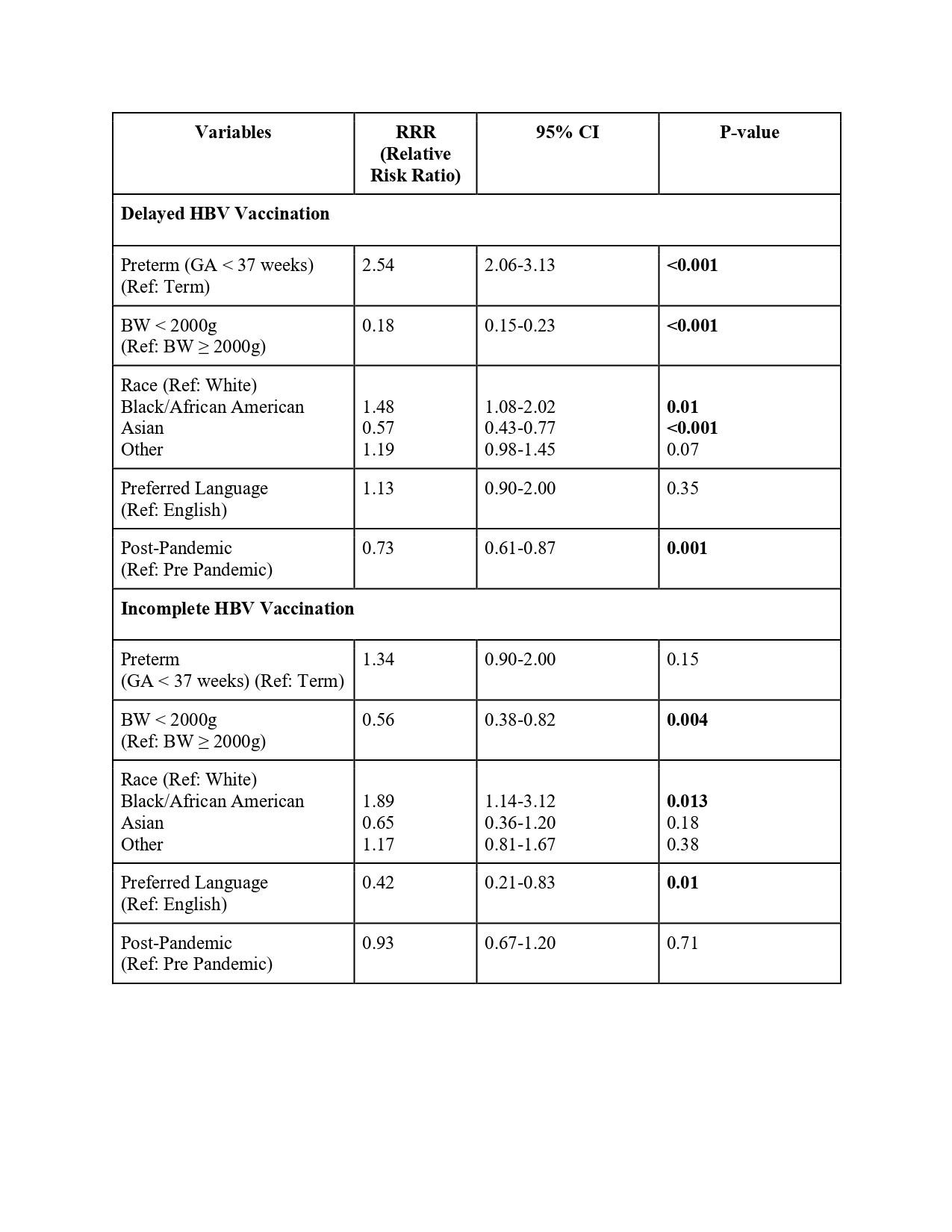
Figure 2: A) Factors associated with delayed or incomplete HBV vaccination, based on 2017 AAP guidance, among infants born at BIDMC and admitted to the NICU between 2018 and 2023. B) Time-to-HBV Vaccination among NICU infants with delayed vaccination from 2018-2023, categorized by Birth Weight (BW).