Neonatal Quality Improvement 2
Session: Neonatal Quality Improvement 2
076 - Outcomes after implementation of Minimally Invasive Surfactant Therapy (MIST) in a Neonatal Intensive Care Unit (NICU)
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 76.5742
Emiko Yamada, Comer Children's Hospital at University of Chicago Medical Center, Chicago, IL, United States; Walid Hussain, Comer Children's Hospital at University of Chicago Medical Center, Chicago, IL, United States; Natasha Ahn, Comer Children's Hospital at University of Chicago Medical Center, Chicago, IL, United States; Kellie Barsotti, Oregon Health & Science University School of Medicine, Portland, OR, United States

Emiko Yamada, MD (she/her/hers)
Resident
Comer Children's Hospital at University of Chicago Medical Center
Chicago, Illinois, United States
Presenting Author(s)
Background: Early surfactant administration is important in treating respiratory distress syndrome (RDS) and reducing rates of bronchopulmonary dysplasia (BPD) in preterm neonates. Minimally invasive surfactant therapy (MIST) delivers surfactant through a thin catheter while the neonate is spontaneously breathing and eliminates the need for intubation and positive pressure ventilation to deliver surfactant. Increasing evidence has demonstrated that use of MIST is associated with lower rates of adverse outcomes including rates of death, BPD and need for intubation and mechanical ventilation.
Objective: Our goal was to examine the effects of implementing MIST on outcomes in comparison to intubation-surfactant-extubation (InSurE) in a NICU where the previous standard of care was InSurE for delivery of noninvasive surfactant. We also compared two timeframes, pre-MIST and post-MIST implementation, to see if there was a difference in outcomes in all patients receiving surfactant, including intubated patients.
Design/Methods: Chart review was completed of all patients who received surfactant (poractant alpha) from June 2022 to May 2024, either from MIST, InSurE or intubation and ventilation in a single center level IV 71 bed NICU. MIST was implemented in June 2023, with timeframe shown in Figure 1. Patients eligible for InSurE were eligible for MIST, which was based on FiO2 requirement on non-invasive ventilation.
Results: There were no significant differences in rates of BPD between the patients who received MIST compared to InSurE (Table 1). Additionally, there were no significant differences in secondary outcomes, including grade III or IV intraventricular hemorrhage (IVH) or periventricular leukomalacia (PVL), air leak requiring drainage, retinopathy of prematurity (ROP) requiring treatment, and necrotizing enterocolitis (NEC) (Table 1). Significantly less patients in the MIST group (1%) received premedication compared to InSurE group (16%) (p=0.003, Table 1). Regarding analysis of the timeframes, significantly more patients (62%) received surfactant noninvasively after implementation of MIST compared to prior to MIST implementation (29%) (p < 0.001, Table 2). There were not any significant changes in rates of BPD or secondary outcomes between the pre-MIST and post-MIST implementation groups (Table 2).
Conclusion(s): After implementation of MIST, significantly more patients received surfactant noninvasively, which over a longer time period is likely to improve respiratory outcomes. However, the lack of significant differences in respiratory and secondary outcomes is likely due to not being powered with enough patients.
Implementation of MIST and percentage of surfactant given via noninvasively
.jpg)
Comparison of MIST versus InSurE outcomes
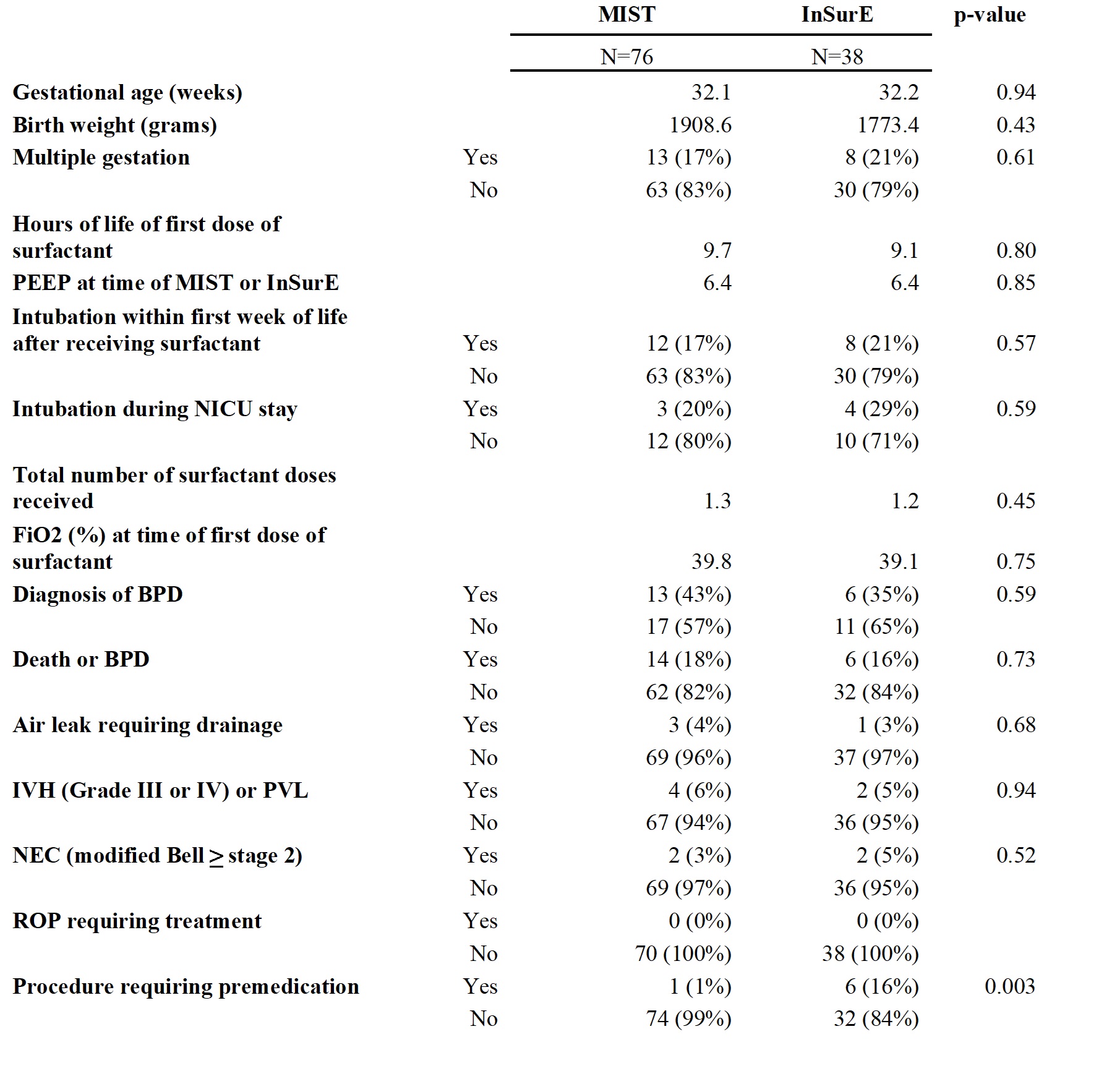 Mean is shown for gestational age, birth weight, hours of life of first dose of surfactant, PEEP at time of MIST or InSurE, total number surfactant doses received and FiO2 (%) at time of first dose of surfactant.
Mean is shown for gestational age, birth weight, hours of life of first dose of surfactant, PEEP at time of MIST or InSurE, total number surfactant doses received and FiO2 (%) at time of first dose of surfactant. Comparison of pre-MIST implementation and post-MIST implementation outcomes
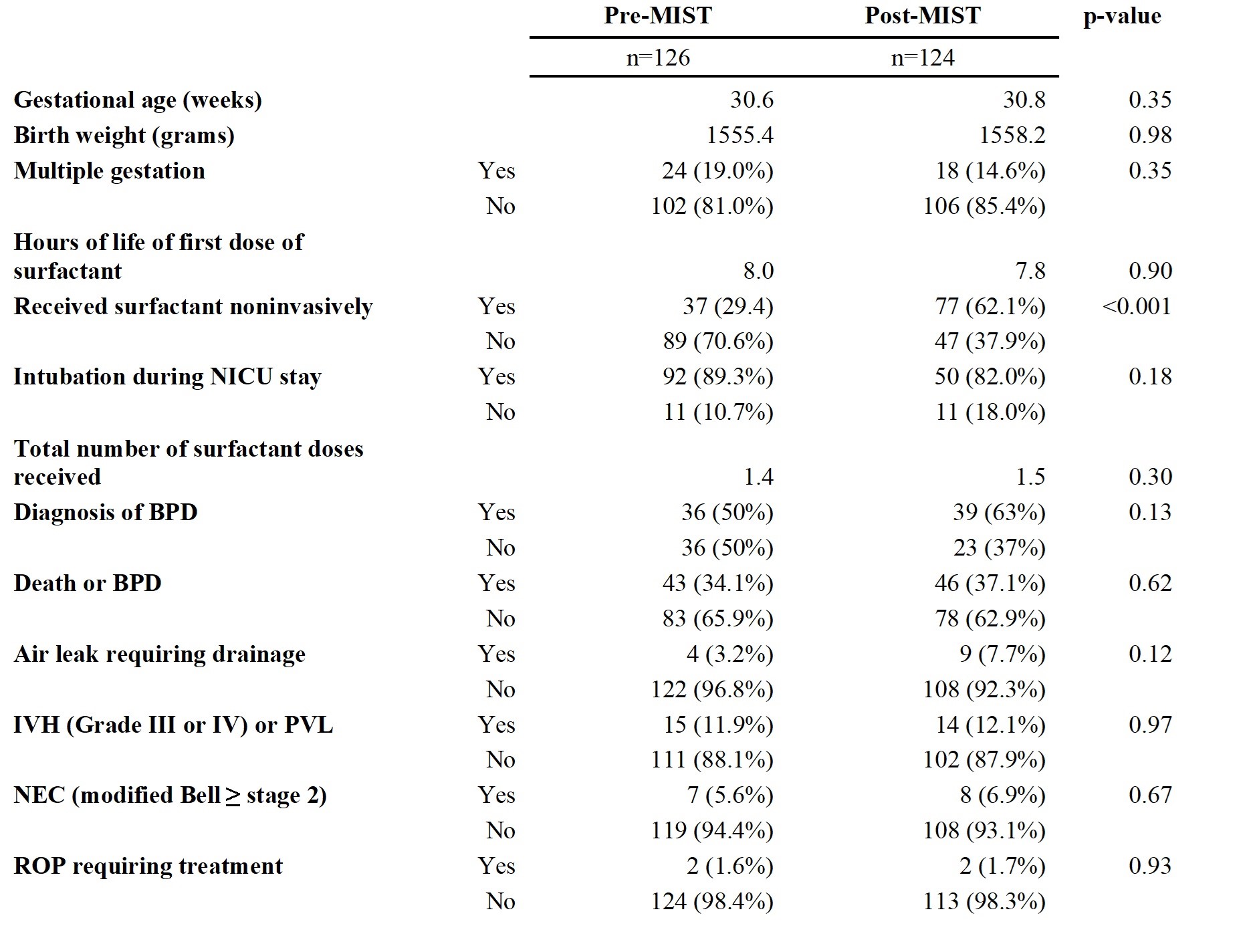 Mean is shown for gestational age, birth weight, hours of life at first dose of surfactant, and total number of surfactant doses received.
Mean is shown for gestational age, birth weight, hours of life at first dose of surfactant, and total number of surfactant doses received.
