Pediatric Therapeutics and Pharmacology
Session: Pediatric Therapeutics and Pharmacology
815 - In Vivo Cytochrome P450 1A2 Activity in Adolescents with and without Obesity
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 815.5499
Jón Trærup Andersen, University of Copenhagen, Copenhagen, Hovedstaden, Denmark; Ulrik Lausten-Thomsen, Department of Intensive Care for Newborns and Infants, Copenhagen University Hospital Rigshospitalet, Copenhagen, Hovedstaden, Denmark; Christina Gade, Department of Clinical Pharmacology, Copenhagen, Copenhagen, Hovedstaden, Denmark
- CG
Christina Gade, PhD, MD (she/her/hers)
Senior Consultant
Department of Clinical Pharmacology, Copenhagen
Copenhagen, Hovedstaden, Denmark
Presenting Author(s)
Background: Obesity-induced pathophysiological complications can significantly alter the pharmacokinetics of certain drugs, particularly through altered hepatic clearance that may be linked to non-alcoholic fatty liver disease (1-6). With the rising prevalence of childhood obesity globally, understanding the impact of obesity on drug-metabolizing enzymes is crucial for optimizing pediatric drug treatment and preventing sub- or supra-therapeutic dosing (3). Cytochrome P450 1A2 (CYP1A2) plays a vital role in drug metabolism, influencing the pharmacokinetics of several important medications used in pediatric practice. Notable examples include theophylline, which is commonly prescribed for asthma and other respiratory conditions, melatonin, used for sleep disorders, and some antipsychotics, e.g. olanzapine (4,7).
Objective: This study aimed to investigate the in vivo activity of CYP1A2 in a larger sample of obese and non-obese children and adolescents aged 11-18 years, as prior research has yielded inconclusive results regarding the impact of obesity on CYP1A2 activity in this population (2,8).
Design/Methods: The study was designed as an open-label, exploratory pharmacokinetic to assess the in vivo activity of CYP1A2 conducted in Denmark (7). Children were recruited based on specific eligibility criteria, including age, biological sex, and body mass index (BMI) Standard Deviation Score (SDS). A total sample size of 64 participants was calculated to detect differences in CYP1A2 clearance. The urinary metabolic ratio (u-MR) of paraxanthine to caffeine was used to evaluate CYP1A2 activity, while hepatic fat content was measured using Magnetic Resonance Spectroscopy (MRS).
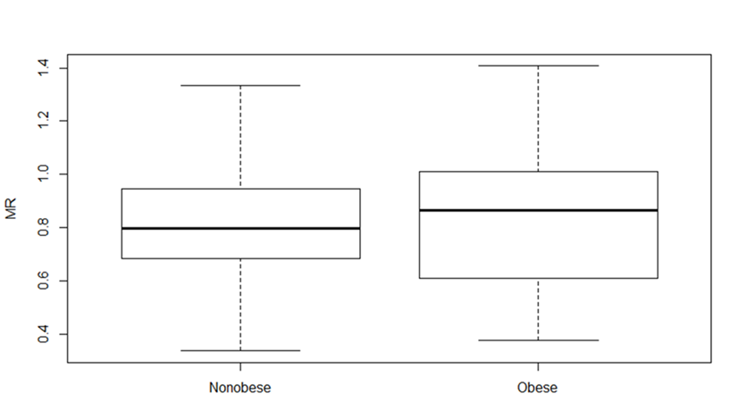
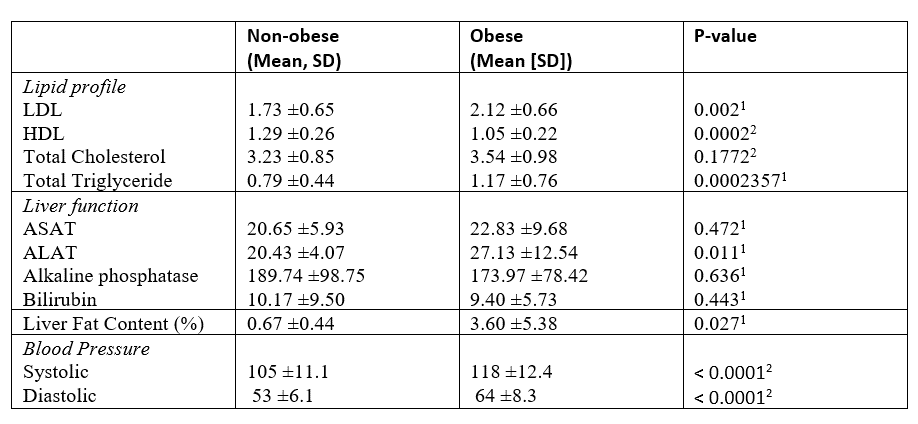
Results: A total of 65 children (31 females, 34 males) completed the study, consisting of 30 children with obesity and 35 non-obese controls (Table 1). Although significant differences were found in liver fat content, lipid profile and blood pressure (Table 1 and 2), no significant differences were observed in the paraxanthine/caffeine u-MR between the two groups (mean Log10 (u.MR): 0.82 vs. 0.82, 95% CI -0.12 to 0.11), see figure1.
Conclusion(s): This study found that obesity does not significantly affect in vivo CYP1A2 activity in children and adolescents aged 11-18 years. These findings suggest that pediatric populations with obesity may not require differential dosing considerations based solely on CYP1A2 activity, though further research is warranted to explore alternate influencing factors on drug metabolism within this demographic.
Table 1. Demographics and clinical characteristics
.png) N: number; BMI SDS: Body Mass Index Standard Deviation Score; 1. binomial test; 2. X2 test; 3. Welch t-test; 4. Mann-Whitney U Test.
N: number; BMI SDS: Body Mass Index Standard Deviation Score; 1. binomial test; 2. X2 test; 3. Welch t-test; 4. Mann-Whitney U Test.Table 2. Biochemistry and MRS results
 1.Mann-Whitney U Test; 2. Welch t-test
1.Mann-Whitney U Test; 2. Welch t-testFigure 1. The metabolic paraxanthine/caffeine ratio for both the mean observed log10 transformed ratios (u-MR) in obese and nonobese children aged 11-18 years. Mean 0.82 versus 0.82, P= 0.93 % CI -0.12- 0.11