Neonatal General 1: Respiratory, BPD
Session: Neonatal General 1: Respiratory, BPD
266 - Metabolic Impact of Extended Continuous Positive Airway Pressure in Stable Preterm Infants: A Secondary Analysis of a Randomized Controlled Trial
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 266.5649
Philip Ballard, UCSF Benioff Children's Hospital San Francisco, Bodega Bay, CA, United States; Kelvin D. MacDonald, Oregon Health and Science University, Lake Oswego, OR, United States; Julia Harris, Oregon Health & Science University School of Medicine, Portland, OR, United States; Thaybeth I.. Malave-Mendez, University of California, San Francisco, School of Medicine, San Francisco, CA, United States; Mitzi Go, Oregon Health & Science University School of Medicine, Portland, OR, United States; Alec Martin, Doernbecher Children's Hospital at Oregon Health & Science University, Portland, OR, United States; Kristin Milner, Oregon Health & Science University School of Medicine, Portland, OR, United States; Robert Tepper, Indiana University School of Mediciane, Indianapolis, IN, United States; Dara G. Torgerson, University of California San Francisco, San Francisco, CA, United States; Cindy T. McEvoy, Oregon Health & Science University School of Medicine, supervisor, OR, United States

Cindy T. McEvoy, MD, MCR (she/her/hers)
Professor of Pediatrics
Oregon Health & Science University School of Medicine
supervisor, Oregon, United States
Presenting Author(s)
Background: Extending the duration of CPAP in stable preterm infants may improve lung growth.
Objective: We assessed the impact of extended (e) CPAP on the metabolic profile.
Design/Methods: Infants born at ≤32 weeks gestational age (GA) and needing ≥24 hours of CPAP were randomized to an extended 2 weeks of bubble CPAP with room air (eCPAP) or to discontinue CPAP to room air (dCPAP) when meeting “CPAP stability criteria”. Functional residual capacity (FRC) was measured at randomization and at the end of the 2-week treatment. All infants received only breast milk and supplements during the 2 weeks except for 14 infants on partial parenteral nutrition for 1-4 days. Blood samples obtained during the treatment interval from 64 infants were subjected to UHPLC:MS/MS (Metabolon Inc.).
Results: The 33 eCPAP and 31 dCPAP infants had similar GA, birth weight, postnatal age at randomization, sex, race/ethnicity, caloric intake and FRC at day 0, but the increases (%) at day 14 in both FRC (2.1-fold, p=0.005) and weight (1.13-fold, p=0.03) were greater in eCPAP infants (Table). For all infants, levels of 46% of 1101 named blood metabolites differed (p < 0.05) by postnatal age at blood collection, which was adjusted for in the analysis. Levels of 72 metabolites (6.5%) were higher in dCPAP than eCPAP infants and 78 metabolites (7.1%) were lower at p< 0.05. A new plateau level over time for most key altered metabolites in dCPAP infants was reached at ≤3 days. Biochemicals with higher levels (mean 1.67-fold) at p< 0.001 in dCPAP infants included 3 bilirubin metabolites, 3 acylcarnitines, dihydroorotate, spermidine, creatine and deoxycarnitine, and those with lower levels (mean 1.72-fold) included 2-aminoheptanonate (homonorleucine), glucuronate, pregnanediol sulfate, nicotinamide riboside, N1-methyladenosine and N-acetyltheanine. Examining the distribution of altered metabolites among 86 biochemical sub pathways that were represented at ≥3 metabolites/sub pathway, enrichment at p< 0.05 occurred for 3 sub pathways: bilirubin synthesis/degradation (7.7-fold, all metabolites higher in dCPAP), fatty acid metabolism-all acyl carnitines (3.5-fold, all higher in dCPAP), and xanthine metabolism (4.5-fold, all lower in dCPAP) (Figure 1) There were significant associations of levels of some highly modified metabolites with changes in FRC at 14 days (Figure 2).
Conclusion(s): CPAP treatment altered the blood metabolome, in part due to hepatic mitochondrial function, indicating that eCPAP may affect nutritional status. eCPAP may reduce the work of breathing allowing more energy to be available for lung and body growth.
Table
.jpg) Table
TableFigure 1. Volcano Plot
.jpg) Volcano plot of results from metabolome-wide association study (MWAS) using ANCOVA comparing blood metabolite levels between stable infants randomized to extended vs. discontinued CPAP (eCPAP -v- dCPAP). Individual dots represent a single metabolite colored by super pathway. Dots above the dashed red line represent metabolites that differed by randomized treatment group at p<0.05. The data shows there are multiple metabolites whose levels are significantly different between eCPAP and dCPAP.
Volcano plot of results from metabolome-wide association study (MWAS) using ANCOVA comparing blood metabolite levels between stable infants randomized to extended vs. discontinued CPAP (eCPAP -v- dCPAP). Individual dots represent a single metabolite colored by super pathway. Dots above the dashed red line represent metabolites that differed by randomized treatment group at p<0.05. The data shows there are multiple metabolites whose levels are significantly different between eCPAP and dCPAP.Figure 2
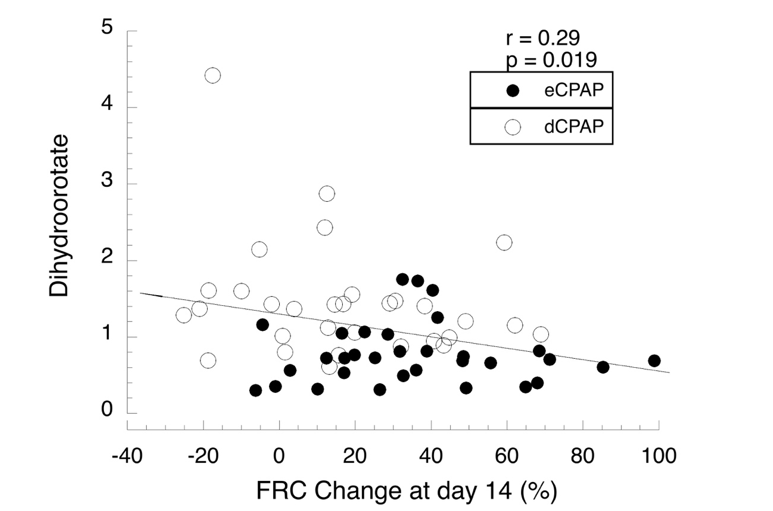 Regression analysis for blood levels of dihydroorotate, a precursor for de novo pyrimidine synthesis vs change in FRC for all 64 infants. Levels of metabolites were compared by ANCOVA and linear regression.
Regression analysis for blood levels of dihydroorotate, a precursor for de novo pyrimidine synthesis vs change in FRC for all 64 infants. Levels of metabolites were compared by ANCOVA and linear regression. Table
.jpg) Table
TableFigure 1. Volcano Plot
.jpg) Volcano plot of results from metabolome-wide association study (MWAS) using ANCOVA comparing blood metabolite levels between stable infants randomized to extended vs. discontinued CPAP (eCPAP -v- dCPAP). Individual dots represent a single metabolite colored by super pathway. Dots above the dashed red line represent metabolites that differed by randomized treatment group at p<0.05. The data shows there are multiple metabolites whose levels are significantly different between eCPAP and dCPAP.
Volcano plot of results from metabolome-wide association study (MWAS) using ANCOVA comparing blood metabolite levels between stable infants randomized to extended vs. discontinued CPAP (eCPAP -v- dCPAP). Individual dots represent a single metabolite colored by super pathway. Dots above the dashed red line represent metabolites that differed by randomized treatment group at p<0.05. The data shows there are multiple metabolites whose levels are significantly different between eCPAP and dCPAP.Figure 2
 Regression analysis for blood levels of dihydroorotate, a precursor for de novo pyrimidine synthesis vs change in FRC for all 64 infants. Levels of metabolites were compared by ANCOVA and linear regression.
Regression analysis for blood levels of dihydroorotate, a precursor for de novo pyrimidine synthesis vs change in FRC for all 64 infants. Levels of metabolites were compared by ANCOVA and linear regression. 
