Neonatal Pulmonology - Clinical 2: BPD: Incidence, Treatment, Outcomes
Session: Neonatal Pulmonology - Clinical 2: BPD: Incidence, Treatment, Outcomes
263 - Postnatal Steroids and Associations with In-Hospital Outcomes for Preterm Infants at the Edge of Viability: A single unit experience
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 263.6348
Madison Lodge, Seattle Children's, Seattle, WA, United States; Robert H. Schmicker, University of Washington, Seattle, WA, United States; Mary Akers, Mary Akers, Edmonds, WA, United States; Amisha P. Tarkunde, University of Washington School of Medicine, Seattle, WA, United States; Claire B. Vietri, University of Washington School of Medicine, Seattle, WA, United States; Tiffany Stanley, University of Washington School of Medicine, seattle, WA, United States; Kendell German, University of Washington School of Medicine, Seattle, WA, United States; Kirtikumar Upadhyay, University of Washington School of Medicine, Seattle, WA, United States; Sarah E. Kolnik, University of Washington - Seattle Children's Hospital, Seattle, WA, United States; Krystle M.. Perez, University of Washington, Seattle, WA, United States
- ML
Madison Lodge, MD (she/her/hers)
PGY-3
Seattle Children's
Seattle, Washington, United States
Presenting Author(s)
Background: Extremely preterm (EP) infants born at the edge of viability [22 to < 25 weeks gestational age (GA)] are often empirically exposed to postnatal steroids to mitigate complications. However, postnatal steroid use may also be associated with adverse gastrointestinal and neurodevelopmental effects. In EP infants, the impact of postnatal steroids on outcomes remains understudied.
Objective: To describe exposure, timing and cumulative dosing of hydrocortisone and dexamethasone among EP infants and associations with in-hospital outcomes in a single level IV NICU.
Design/Methods: We performed a retrospective analysis of all EP admissions from April 2021 to March 2024. Descriptive statistics were utilized for maternal-infant factors. Type, timing of exposure, and cumulative dosing of postnatal steroids were collected. Fisher’s exact test evaluated postnatal steroid exposure association with specified NICU outcomes
Results: 61 EP infants were born during the period of the study. 50 (82%) survived at least 72 hours and 32 (52%) survived through hospital discharge. Median GA was 23.7 weeks (Range 22.0, 24.8) and median birthweight was 600 grams (IQR: 530, 660) and there were no differences between EP infants surviving to discharge and those that died (Table 1). 59 (97%) EP infants received early hydrocortisone with median initiation at postnatal day 0 (0,1) and median cumulative dose 14.9 mg/kg (9.6, 27.9). Additionally, 22 (36%) EP infants received at least 10-days of dexamethasone (Table 2). Increasing hydrocortisone cumulative dosing was not associated with an increased risk of stage 2+ necrotizing enterocolitis (NEC) or spontaneous intestinal perforation (SIP); increasing hydrocortisone was also not associated with decreased risk of stage 2+ bronchopulmonary dysplasia (BPD) (Table 3). However, for every unit increase of cumulative hydrocortisone dose, there was increased risk of having a patent ductus arteriosus requiring catheter closure (cPDA) (aOR 16.4, 95%CI: 1.18, 226). Exposure to hydrocortisone or dexamethasone was not associated with any of the select in-hospital outcomes in this small cohort (Table 3).
Conclusion(s):
Conclusion: Most EP infants are exposed to postnatal steroids. There was a significant association with increasing hydrocortisone dosing and cPDA. More research is needed to quantify the possible risk-benefit ratio of exposure to moderate-long-term outcomes in the EP population.
Table 1: Demographic Information from N=50 EP infants surviving at least 72 hours (maternal characteristics, complications of labor, and infant characteristics)
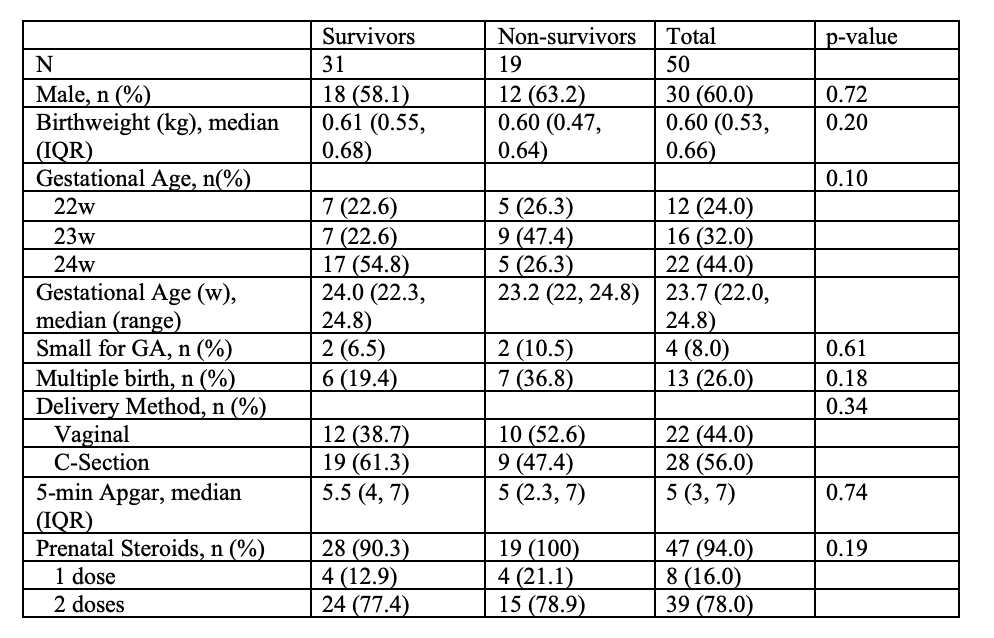
Table 2: Descriptive information of steroid timing, duration and cumulative dose stratified by gestational age.
.png) *Course was a 10-day course
*Course was a 10-day course #Percentage is shown as percentage of those surviving at least 72 hours
Table 3: Clinical outcomes associated with hydrocortisone and dexamethasone dosing.
.png) Adjusted Odds ratios (OR) are provided for every unit increase of cumulative hydrocortisone the odds of clinical outcome as compared to the absence of the outcome. aOR are adjusted for birthweight and gestational age at birth.
Adjusted Odds ratios (OR) are provided for every unit increase of cumulative hydrocortisone the odds of clinical outcome as compared to the absence of the outcome. aOR are adjusted for birthweight and gestational age at birth.*The 95%CI is from an logistic regression model where Hydrocortisone is log transformed to deal with skewedness.
#Excluding n=1 infant discharged with a Gastrostomy tube for home
^Excluding the n=1 infant discharged with a Tracheostomy
Abbreviations are as follows: NEC Necrotizing Enterocolitis; SIP: Spontaneous Intestinal Perforation; cPDA: Patent ductus arteriosus requiring catheter closure; BPD: Bronchopulmonary Dysplasia; ROP: Retinopathy of Prematurity; NG: Nasogastric

