Neonatal General 1: Respiratory, BPD
Session: Neonatal General 1: Respiratory, BPD
278 - Single center experience exploring NICU outcomes after initiating routine early use of hydrocortisone extremely low birthweight infants
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 278.6667
Tejeshwar S. Sangha, Eastern Virginia Medical School, Norfolk, VA, United States; Carlos Rodrigo Castro Castaneda, Children's Hospital of The King's Daughters, Norfolk, VA, United States; Hannah Jolls, Eastern Virginia Medical School, Norfolk, VA, United States; Brittany Erwin, Children's Hospital of The King's Daughters, Suffolk, VA, United States; Turaj Vazifedan, Eastern Virginia Medical School, Norfolk, VA, United States; Haree K. Pallera, Children's Hospital of The King's Daughters / Eastern Virginia Medical School, Norfolk, VA, United States; Tushar A. Shah, Children's Hospital of The King's Daughters, Norfolk, VA, United States

Tejeshwar S. Sangha, MD (he/him/his)
Resident PGY2
Eastern Virginia Medical School/ODU Health Sciences
Norfolk, Virginia, United States
Presenting Author(s)
Background: Bronchopulmonary Dysplasia (BPD) is a leading cause of morbidity and mortality in preterm infants. Recent trials (STOP-BPD, PREMILOC) have shown that hydrocortisone increased survival without BPD at 36 weeks postmenstrual age (PMA). The NICU at the Children’s Hospital of the King’s Daughters (CHKD) has been using hydrocortisone for BPD prevention based on the PREMILOC protocol since 2017.
Objective: To measure the impact of early hydrocortisone use in preterm infants on BPD and other NICU outcomes.
Design/Methods: A retrospective chart review was conducted on infants ≤ 29 WGA and/or birth weight ≤ 1000g, who received hydrocortisone for BPD prevention admitted to the CHKD NICU from June 2017-2019 (n=190). Infants born prior to initiation of the protocol from 2015-2017 were used as controls (n=199). The primary outcome measured was survival without BPD at 36 weeks PMA. Quantile regression model was used to compare the cumulative hydrocortisone dose pre-versus-post-intervention. A logistic regression model was used to assess the impact of implementing protocol on the primary and secondary outcomes, adjusted for the effect of confounders. Odds Ratio (OR) and 95% adjusted odds ratio (aOR) were computed using SPSS 28 (Chicago, IL)
Results: Demographic characteristics are summarized in Table 1. In univariate analysis, the likelihood of cumulative dose of hydrocortisone ≥ 9mg/kg was significantly lower post-intervention, OR=0.29, 95% CI (0.18, 0.48), p< 0.001). The odds of requiring vasopressors for more than 48 total hours in the first week of life was significantly higher pre-intervention, OR= 2.16, 95% CI (1.06, 4.48), p=0.033 (Table 2). In multivariate logistic regression analysis, however, implementing the protocol did not decrease the likelihood of survival without BPD, aOR=0.63, 95% CI (0.38, 1.07), p=0.09. The odds of BPD significantly increased if PDA required pharmacologic or surgical intervention (aOR=2.01, 95% CI (1.07, 3.77), p=0.030) or infant had late onset sepsis (aOR=2.26, 95% CI (1.26, 4.05), p=0.006), irrespective of hydrocortisone use (Table 3). There were no significant differences in the incidence of late onset sepsis, IVH, PDA requiring intervention, ROP or SIP/NEC post-intervention, when compared to controls.
Conclusion(s): While implementation of routine early use hydrocortisone decreased cumulative dose of hydrocortisone and vasopressor use, there was no significant difference in the incidence of survival without BPD post-intervention. Future steps include tracking long-term neurodevelopmental outcomes at 1-2 years after discharge.
Table 1
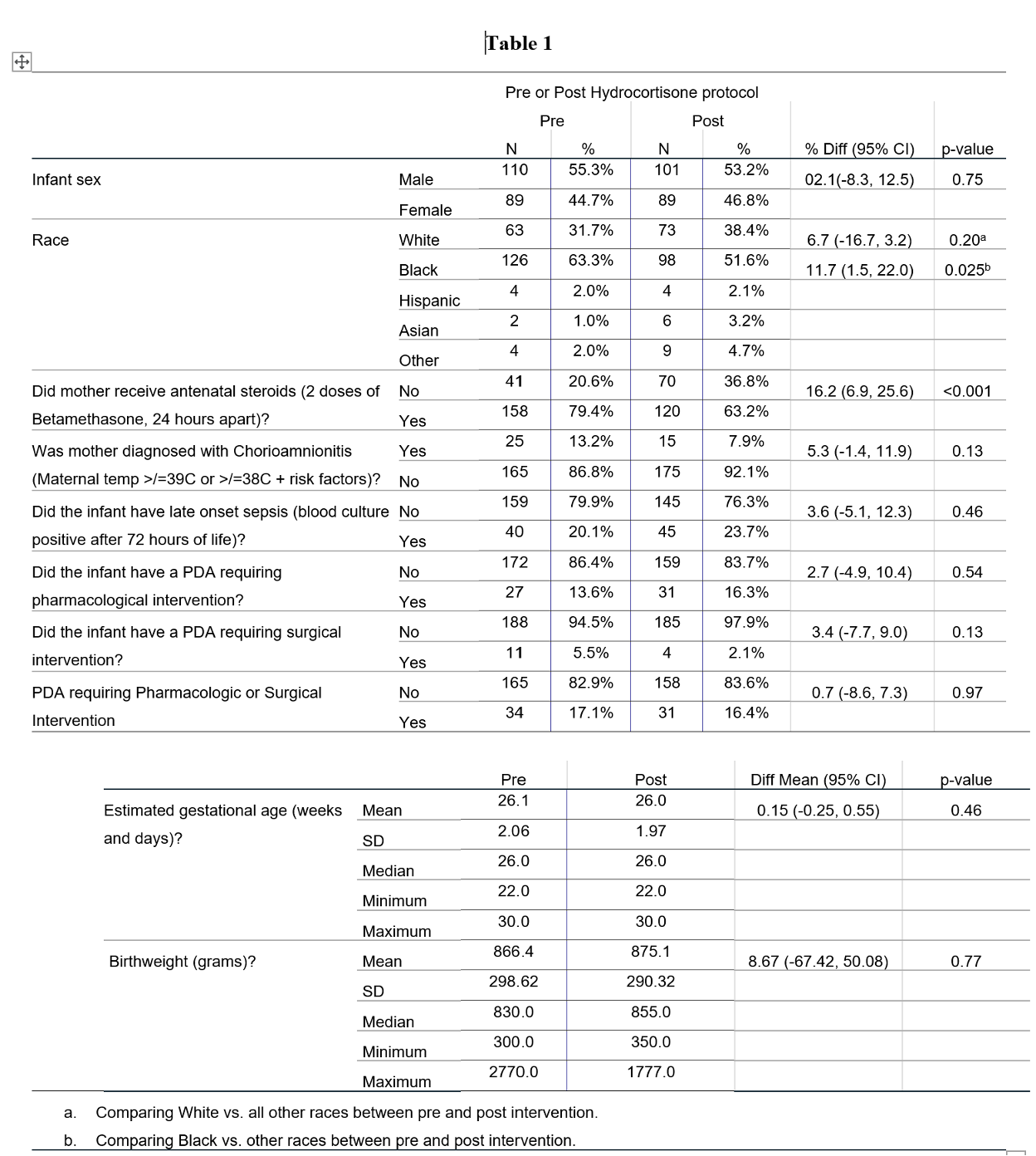 Baseline Characteristics
Baseline CharacteristicsTable 2
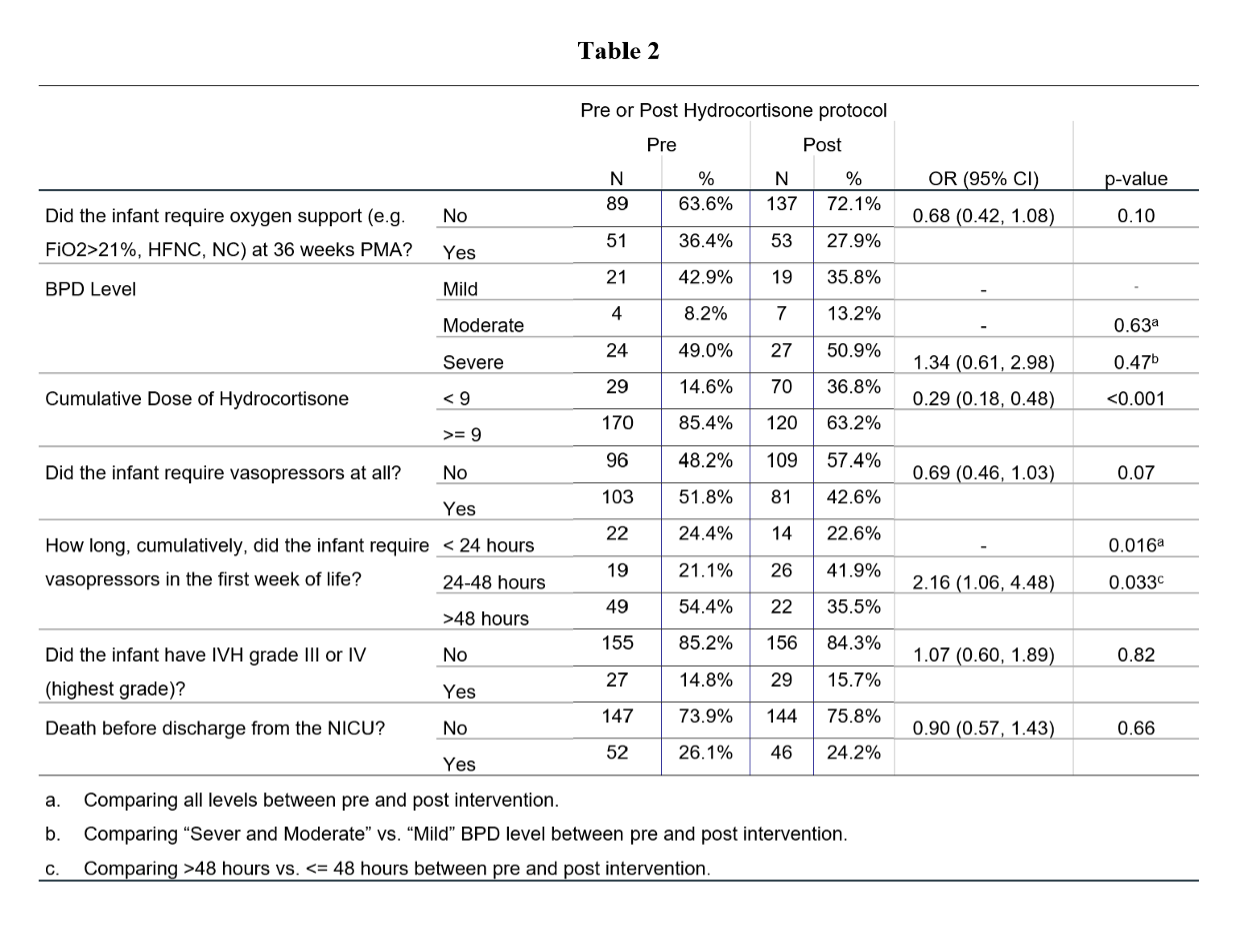 Primary outcomes
Primary outcomesTable 3
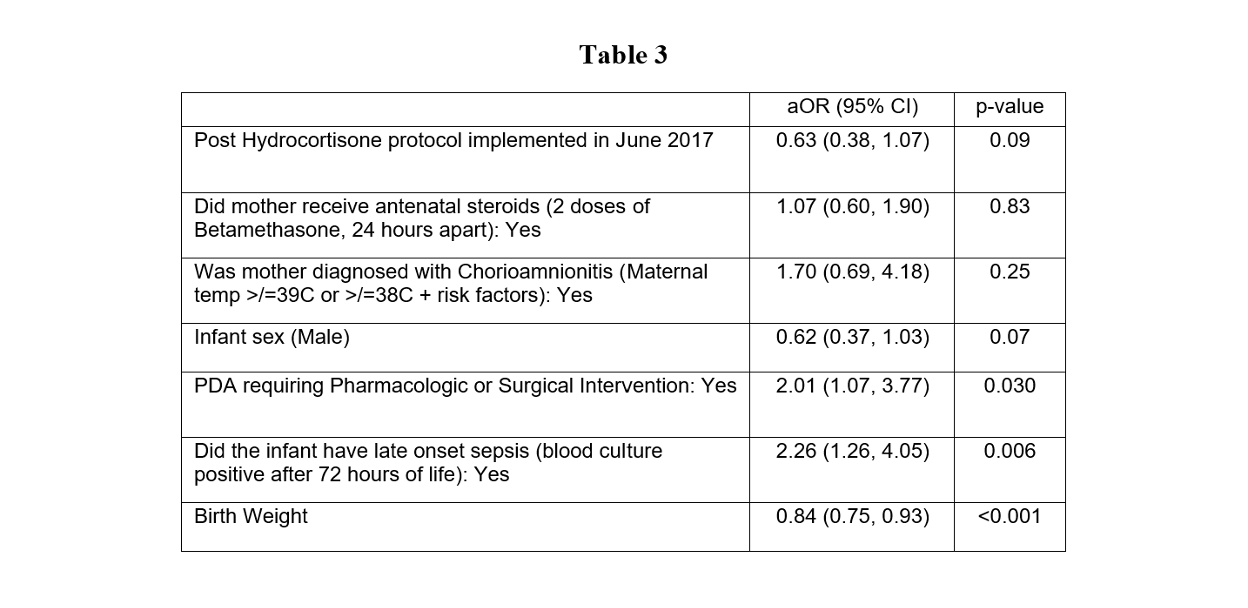 Odds of being diagnosed with BPD
Odds of being diagnosed with BPD
