Neonatal Neurology 2
Session: Neonatal Neurology 2
357 - The role of cerebral perfusion in glycemia-related neurodevelopmental outcomes
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 357.6813
Carmen Monthe-Dreze, Brigham and Women's Hospital, Boston, MA, United States; Sara Cherkerzian, Harvard Medical School, Boston, MA, United States; Ayanna Coburn-Sanderson, Brigham and Women's, Somerville, MA, United States; Isabella Lawandy, Women & Infants Hospital of Rhode Island, Somerville, MA, United States; Laurie Foster, Brigham & Women's Hospital, Boston, MA, United States; Rana Abdel-Rahman, CDC, Atlanta, GA, United States; Camille E. Powe, Massachusetts General Hospital, Boston, MA, United States; Elizabeth Rosenfeld, Children's Hospital of Philadelphia, Philadelphia, PA, United States; Michael SD. Agus, Boston Children's Hospital, Harvard Medical School, Boston, MA, United States; Sarah Lassey, Brigham and Women's Hospital, Boston, MA, United States; Mohamed El-Dib, Harvard Medical School - Brigham and Women's Hospital, Boston, MA, United States; Alfonso Galderisi, Yale School of Medicine, New Haven, CT, United States; Sarbattama Sen, Women & Infants Hospital of Rhode Island, providence, RI, United States

Carmen Monthe-Dreze, MD (she/her/hers)
Neonatologist/Instructor in Pediatrics
Brigham and Women's Hospital/Harvard Medical School
Boston, Massachusetts, United States
Presenting Author(s)
Background: Neurosensory impairment in children with neonatal hypoglycemia (NH), which affects 50% of newborns with risk factors, may be related to steeper initial rise in interstitial glucose (IG) concentrations and higher glucose variability (GV). Vascular compensations to glycemic flux and oxidative stress-mediated neuronal injury have been hypothesized to contribute to outcomes. However, little is known on the role of cerebral tissue perfusion in glycemia-related neurodevelopmental outcomes (NDO).
Objective: Determine associations between neonatal interstitial glucose (IG) patterns and neurodevelopment and examine the moderating role of cerebral perfusion.
Design/Methods: Infants (n=34) ≥ 35-week gestation without significant neonatal morbidities were enrolled in the ongoing observational prospective longitudinal LAMMBS study in Boston, MA. Following delivery, we placed blinded continuous glucose monitors (CGM) to measure IG and cerebral near infrared spectrometry (cNIRS) to measure cerebral oxygenation (CrSaO2), a marker of cerebral perfusion. NDO measures included: (1) NeoNatal Neurobehavioral Scale (NNNS) scores (primary outcome) assessed prior to nursery discharge, and Ages and Stages Questionnaire (ASQ) scores at (2) 6-months, and (3) 12-months. We fit linear regression models to examine associations of (1) mean IG and (2) IG coefficient of variability (CV) with NDO, and included interaction terms to examine modification by %time with CrSaO2 >85%CrSO2 and mean CrSaO2.
Results: Cohort characteristics are shown in Table 1. Mean±SD gestational age was 38.4 ±1.2 weeks and 38% were female. Mean ± SD CGM and cNIRS monitoring duration were 53 ± 19 and 14 ± 10 hours, respectively. Higher mean IG was associated with lower NNNS quality of movement (ẞ -0.03, 95% CI:-0.06, -0.004), and greater glucose variability (IG CV) was associated with lower NNNS self-regulation scores (ẞ -0.2, 95%CI:-0.3, -0.05, Table 2). Higher total ASQ scores were associated with greater glucose variability. Associations between mean IG and quality of movement scores were stronger among infants who spent more time with CrSaO2 > 85% (P-int= 0.09, Fig A). Associations between IG CV and self-regulation scores were stronger with increasing CrSaO2 (P-int=0.15, Fig D).
Conclusion(s): Both greater IG and greater glucose variability in the neonatal period were associated with worse neurobehavior. Higher cerebral perfusion potentiates these associations and may serve as a marker for clinically meaningful NH. Future studies should examine the utility of cNIRS to identify neonates at risk of NH-associated long-term NDO.
Table 1: Participant Characteristics
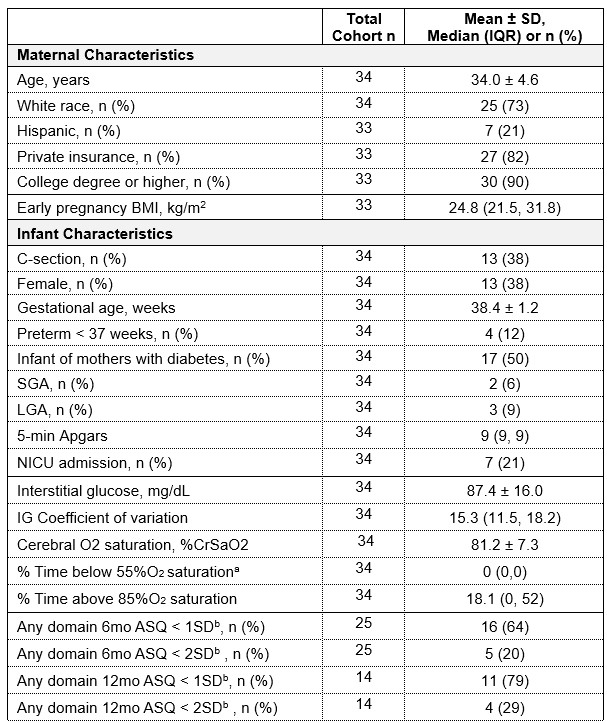 Data presented as mean ± SD for normally distributed variables, median (IQR) for non-normally
Data presented as mean ± SD for normally distributed variables, median (IQR) for non-normallydistributed variables or n (%) for categorical variables. Cohort sample size defined as infants with
non-missing IG data who had any NDO data available. Abbreviations: BMI, body mass index; SD,
standard deviation; IQR, interquartile range; SGA, small-for-gestational age; LGA, large-for-
gestational age; NICU, neonatal intensive care unit; IG, neonatal interstitial glucose; ASQ, Ages
and Stages Questionnaire.
a Min-Max for %time below 55% is 0-2%
b Suspected developmental delay in at least 1 domain based on two cutoffs ( <1SD or <2SD)
Table 2: Associations between neonatal interstitial glucose and infant neurodevelopment
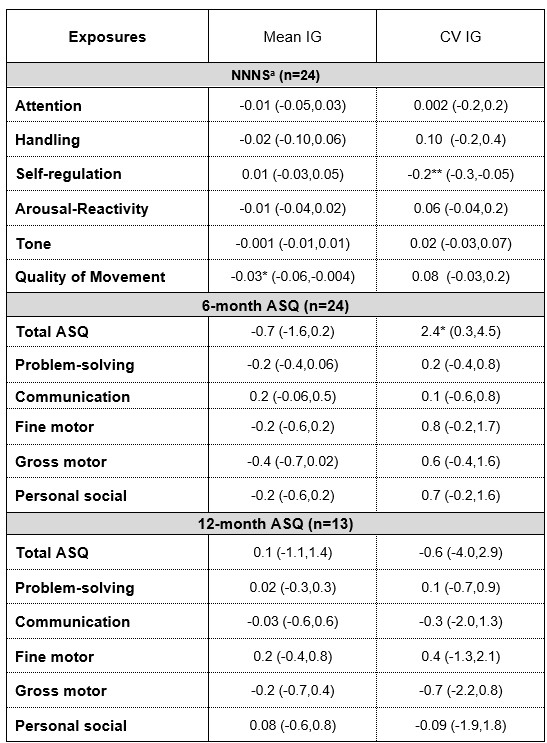 Data are reported as beta estimates (95%CI) from linear regression models adjusted
Data are reported as beta estimates (95%CI) from linear regression models adjusted for initial maternal BMI; gestational age; (+infant sex for NNNS and 6 mo ASQ models).
Abbreviations: IG: interstitial glucose; CV: coefficient of variation; NNNS: NeoNatal
Neurobehavioral Scale; ASQ: Ages and Stages Questionnaire.
a Higher scores = less favorable NNNS measures of handling, arousal/reactivity and tone.
Lower scores = less favorable NNNS measures for attention, self-regulation and
quality of movement.
*P < 0.05; **P < 0.01
Figure: Moderating role of cerebral oxygenation on associations between neonatal interstitial glucose and neurodevelopment.
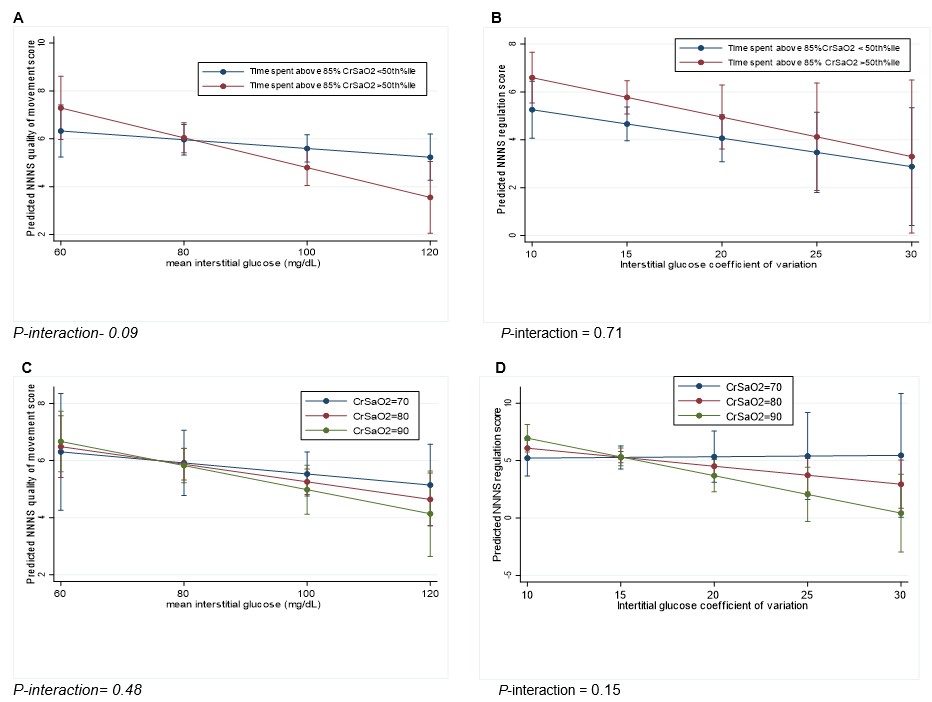 Results are displayed as estimated means with 95%CI from linear regression analyses adjusted for maternal BMI, gestational age and sex, and included a cNIRS measure*IG interaction term. Abbreviation: CrSaO2: Cerebral O2 saturation. Figures A and B: Associations between mean IG and NNNS quality of movement scores (A) and between IG coefficient of variation and NNNS regulation scores (B) stratified by %time CrSaO2 > 85%O2 category (above or below median). Figures C and D: Associations between mean IG and NNNS quality of movement scores (C) and between IG coefficient of variation and NNNS regulation scores (D) while holding CrSaO2 at three different values: 70, 80, and 90%. P-value for interaction was considered significant at P < 0.2.
Results are displayed as estimated means with 95%CI from linear regression analyses adjusted for maternal BMI, gestational age and sex, and included a cNIRS measure*IG interaction term. Abbreviation: CrSaO2: Cerebral O2 saturation. Figures A and B: Associations between mean IG and NNNS quality of movement scores (A) and between IG coefficient of variation and NNNS regulation scores (B) stratified by %time CrSaO2 > 85%O2 category (above or below median). Figures C and D: Associations between mean IG and NNNS quality of movement scores (C) and between IG coefficient of variation and NNNS regulation scores (D) while holding CrSaO2 at three different values: 70, 80, and 90%. P-value for interaction was considered significant at P < 0.2. 
