Neonatal Neurology 3
Session: Neonatal Neurology 3
363 - Updating the systematic review of therapeutic hypothermia (TH) for hypoxic ischemic encephalopathy (HIE)
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 363.6749
Marie T. Berg, University Of Florida, University Of Florida College Of Medicine, FL, United States; Peter Davis, Royal Women's Hospital, Melbourne, Victoria, Australia; Terrie Inder, Childrens Hospital of Orange County, Orange, CA, United States; Rod W. hunt, Monash University, CLAYTON, Victoria, Australia; Susan Jacobs, Royal Women's Hospital, Parkville, Victoria, Australia; William O. Tarnow-Mordi, University of Sydney NHMRC CTC, Sydney, New South Wales, Australia; Roger Soll, Robert Larner, M.D., College of Medicine at the University of Vermont, Hinesburg, VT, United States

Marie T. Berg, MD (she/her/hers)
Clinical Associate Professor
University Of Florida
University Of Florida College Of Medicine, Florida, United States
Presenting Author(s)
Background: In high income countries, therapeutic hypothermia (TH) is now the standard of care for term infants with moderate to severe encephalopathy. Recent randomized controlled trials (RCTs) of TH have focused on novel methods of cooling (phase changing material) in different settings (including low- and middle-income countries).
Objective: To incorporate recent RCTs into a systematic review of TH using GRADE methodology.
Design/Methods: We conducted an electronic search for RCTs comparing hypothermia to normothermia in infants with presumed HIE. HIE was defined by the presence of perinatal asphyxia and the presence of encephalopathy. Methods of cooling included selective head or whole-body cooling with servo control, or the use of ice or phase changing materials. Critical outcomes included death or neurodevelopmental disability (NDD) at 18 to 24 months, mortality, and NDD. We assessed studies using the GRADE criteria (evaluating overall risk of bias, consistency of effect, imprecision, indirectness and publication bias).
Results: 3025 records were identified, of which 130 reports from 29 RCTs were included. Nineteen of these RCTs were not included in the previously published Cochrane analysis.
Analysis of data from all eligible RCTs demonstrated a reduction in death or NDD at 18 to 24 months, mortality, and NDD in infants receiving TH (Table 1). However, an analysis of the funnel plot of death revealed marked asymmetry (Figures 1 and 2). Outlying studies were dominated by recent single center studies utilizing phase changing materials. Asymmetry was not noted in the analyses of NDD, as these were dominated by the larger prior multicenter trials.
The sensitivity analysis, excluding single center reports, revealed similar estimates of the effects on death or NDD or on NND alone to those previously reported (Table 1). However, the more restricted analysis demonstrated weaker evidence of an effect on mortality (RR 0.88, 95% CI 0.77 to 1.01, I2 50%; RD -0.04, 95% CI -0.08 to 0.00, I2 53%). This analysis provides little information regarding the impact of TH outside of the high-income countries where most of these studies were conducted.
Conclusion(s): Infants undergoing TH remain likely to have reduced risk of death or NND. When restricting to multicenter studies, there were far fewer trials of infants from LMICs. Given the demonstrated concern for potential bias in the single center studies using phase changing materials, further analysis is needed to assess these published cooling trials. An individual patient meta-analysis may be helpful in further evaluating potential biases.
Table 1.
.png)
Figure 1. Funnel plot
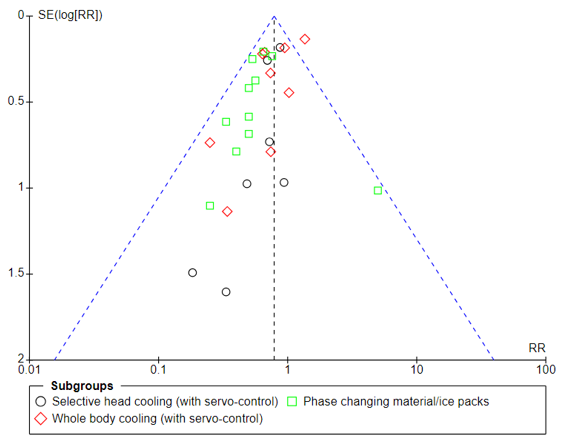 Mortality reported at last follow-up in all infants (all trials)
Mortality reported at last follow-up in all infants (all trials)Figure 2. Funnel plot
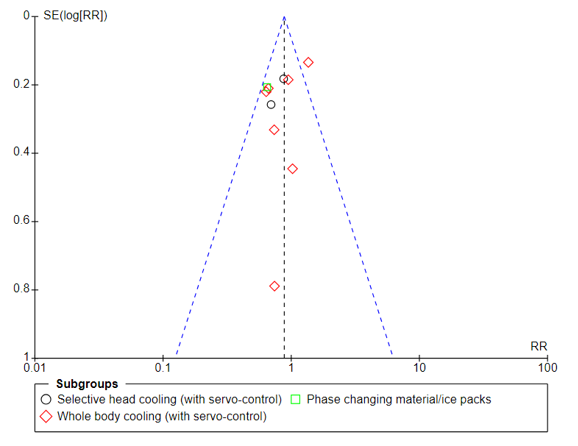 Mortality reported at last follow-up in all infants (multicenter trials)
Mortality reported at last follow-up in all infants (multicenter trials)
