Hematology/Oncology 1: Pediatric Oncology
Session: Hematology/Oncology 1: Pediatric Oncology
102 - Outcomes after Implementation of an Emergency Department Fever and Neutropenia Protocol in Pediatric Oncology Patients
Saturday, April 26, 2025
2:30pm - 4:45pm HST
Publication Number: 102.5973
Nibal A. Eid, Children's Hospital of Michigan, Detroit, MI, United States; Sana Ashraf, Childrens Hospital of Michigan, Detroit, MI, United States; Madison A. Graifman, Wayne State University School of Medicine, Detroit, MI, United States; Danielle Bell, Central Michigan University, Berkley, MI, United States; Rajan Arora, Children's Hospital of Michigan, Central Michigan University, Detroit, MI, United States; Nirupama Kannikeswaran, Central Michigan University College of Medicine, Detroit, MI, United States; Stephanie A. Toll, Children’s Hospital of Michigan, Detroit, MI, United States; Erin Goode, Childrens hospital of michigan, Detroit, MI, United States
- EG
Erin Goode, DO, MPH (she/her/hers)
Pediatric Hematology/Oncology
Children's Hospital of Michigan
Detroit, Michigan, United States
Presenting Author(s)
Background: Febrile neutropenia is a common complication in patients on myelosuppressive chemotherapy. High-Risk criteria have correlated with increased rates of bacteremia and clinical complications. In other disease groups, studies have shown CRP and procalcitonin levels as effective markers in predicting bacterial illnesses. The utility of markers in risk stratification of patients with febrile neutropenia has not been studied.
Objective: Primary objective was to evaluate patient disposition and outcomes after implementation of a clinical practice guideline (CPG) in the ED for management of children with febrile neutropenia. Secondary objectives were to assess the utility of CRP and procalcitonin in prediction of bacteremia in this cohort.
Design/Methods: A tertiary care, free standing children’s hospital created a CPG to standardize evaluation and admission guidelines in febrile oncology and bone marrow transplant patients (Figure 1). Practice prior to this intervention was that all oncology patients with fevers were admitted for 48-hour evaluation. The first PDSA cycle was implemented in January 2024. An EMR order set was implemented May 2024. Demographics, results, disposition and rates of bacteremia were collected.
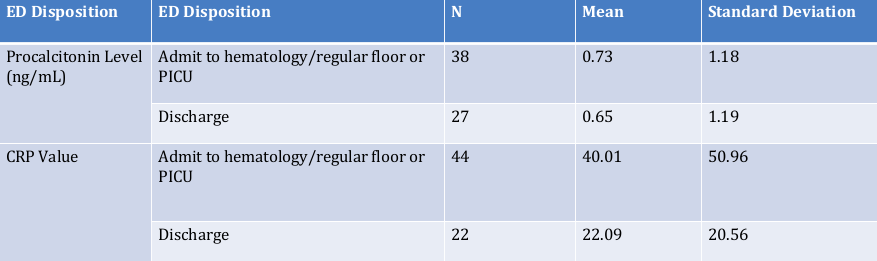
Results: There were 101 emergency room visits for 51 pediatric oncology patients with fever over 9 months. The majority were male (35, 69%), with mean age of 8.26 years (SD 5.47 years). Most patients have liquid tumors (61, 60%). Sixty-six required admission; 16 (31%) had high risk features and 39 patients were neutropenic. Thirty-four were discharged from the Emergency Department (Figure 2) and 18 (17.8%) met discharge criteria but were admitted. Nine required PICU admissions, with one death. There were seven positive central blood cultures, four of which matched positive peripheral cultures. All patients with positive blood cultures were designated as high-risk, requiring admission. The mean CRP was 34.03, and procalcitonin was 0.69. Logistic regression analysis showed that neither CRP (p=0.5) nor procalcitonin(p=0.87) predicted bacteremia, though both levels were higher in admitted children.
Conclusion(s): Implementation of a CPG resulted in reduction in hospitalization without adverse outcomes in low-risk febrile oncology patients. CRP and procalcitonin levels do not seem to predict bacteremia in febrile oncology patients. Additional data to evaluate the utility of these biomarkers in febrile oncology patients is needed.
Figure 1
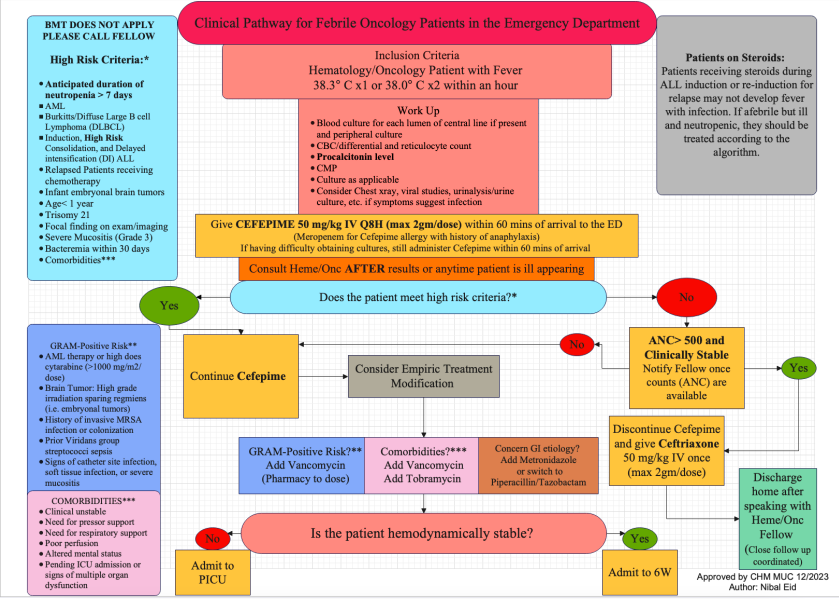
Figure 2