Pediatric Nutrition
Session: Pediatric Nutrition
742 - Parental Health-Related Quality of Life in Response to Child Health Conditions: A Potential Key Factor in Pediatric Tube-Weaning Engagement
Saturday, April 26, 2025
2:30pm - 4:45pm HST
Publication Number: 742.5915
Alexandra M. Golik, University of Kansas Medical Center, Miami, FL, United States; Taylor Harris, Children's Mercy Hospitals and Clinics; University of Kansas Medical Center, Lawrence, KS, United States; Anna Wallisch, University of Kansas Medical Center, Kansas City, KS, United States; E Zhang, University of Kansas Medical Center, Kansas City, KS, United States; Kelsey Dean, University of Kansas School of Medicine, Kansas City, KS, United States; Dana Bakula, Children's Mercy Hospitals and Clinics, Kansas City, MO, United States; Megan Olalde, University of Kansas Medical Center, Kansas City, KS, United States; Amanda Bruce, University of Kansas School of Medicine, Kansas City, KS, United States; Ann M. Davis, University of Kansas Medical Center, KC, KS, United States; Hayat Mousa, Mousa, Newtown Square, PA, United States
- AG
Alexandra M. Golik (she/her/hers)
Graduate Student
University of Kansas Medical Center
Miami, Florida, United States
Presenting Author(s)
Background: Pediatric feeding disorder (PFD) affects 1/37 children, some of whom need feeding tubes due to inadequate oral intake. While lifesaving, tube-feeding can disrupt family functioning and quality of life, and weaning challenges are common. Parents of children with PFD often face increased mental health concerns, which may hinder treatment adherence.
Objective: Examine how child health conditions impact parent health-related quality of life and family functioning/impact (HRQLFI) and their influence on withdrawal rates in a pediatric tube-weaning trial (NCT03815019,NIH R01HD093933), aiming to improve retention and weaning success.
Design/Methods: Sixty-one children (ages 9 months-9 years), receiving >80% of nutrition via gastrostomy tubes, enrolled with parents at 8 hospitals for the iKanEat trial. The 24-week trial included biweekly telehealth coaching, 4 medical visits, a 10-day tube-feed reduction (Week 10), and randomization to megestrol or placebo. Parents completed a baseline PedsQL to assess the impact of their child's health on their HRQLFI. Lower scores indicate poorer outcomes. Mean scores were compared between families who withdrew and those who remained, with trends examined due to the small sample size.
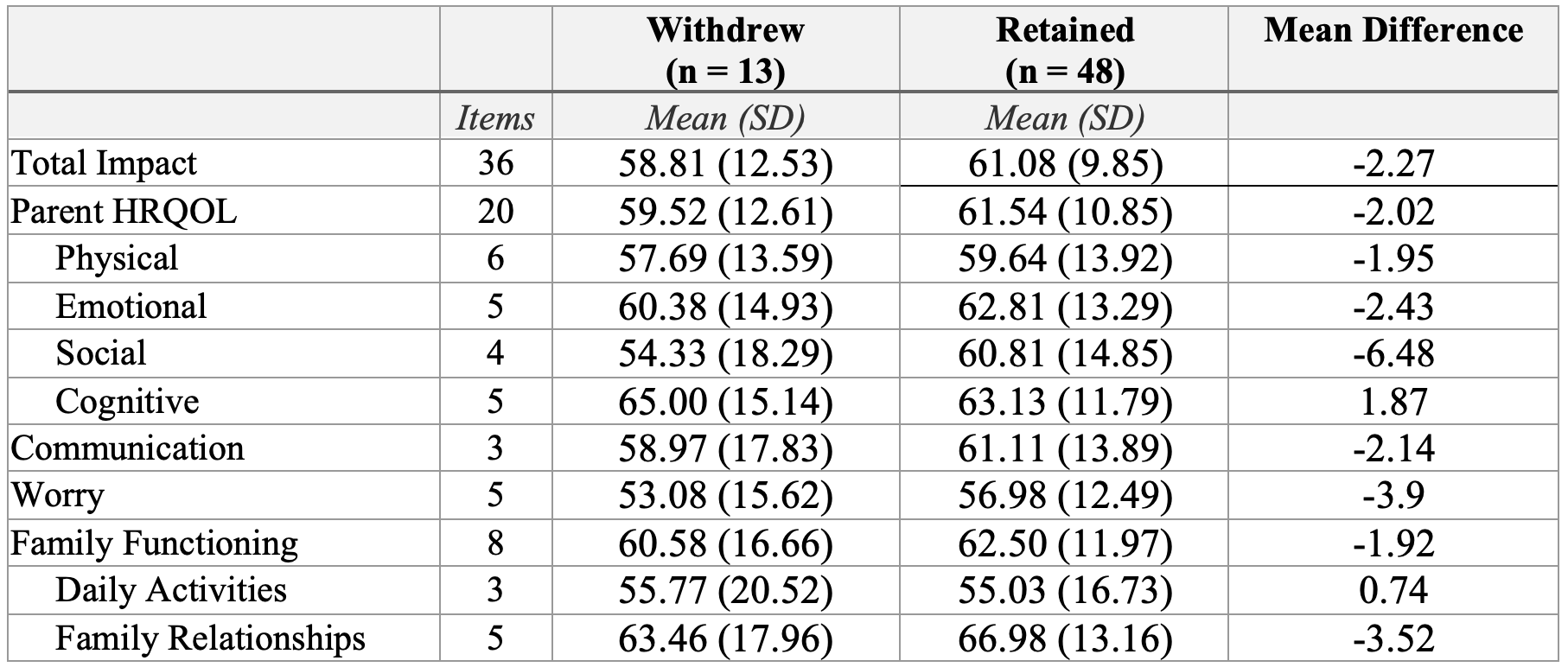
Results: Thirteen (21.3%) of 61 families withdrew. Withdrawing parents reported lower scores in overall impact (M=58.81 vs. 61.08), parent HRQOL (M=59.52 vs. 61.54), physical (M=57.69 vs. 59.64), emotional (M=60.38 vs. 62.81), social (M=54.33 vs. 60.81), communication (M=58.97 vs. 61.11), worry (M=53.08 vs. 56.98), family functioning (M=60.58 vs. 62.50), and family relationship (M=63.46 vs. 66.98) domains, with the largest difference in social functioning (6.48 points lower). Exceptions included slightly higher cognitive functioning scores for withdrawing families (M=65.00 vs. 63.13) and comparable daily activity scores (M=55.77 vs 55.03) across groups.
Conclusion(s): A 21.31% withdrawal rate highlights the challenges of pediatric tube-weaning. Withdrawing parents reported poorer functioning and quality of life across physical, emotional, social, overall parent HRQOL, communication, worry, family functioning, family relationships, and overall impact domains prior to the tube-weaning intervention, compared to parents who remained. Findings suggest parent HRQLFI may impact adherence to pediatric tube-weaning. Supporting parent HRQLFI may improve motivation, lower withdrawal rates, and increase pediatric weaning success, ultimately enhancing quality of life for both children and their families. Further implications and directions will be discussed.
Table 1. Parent PedsQL Scores, Mean Differences for Withdrawal vs. Retained Families