Health Equity/Social Determinants of Health 2
Session: Health Equity/Social Determinants of Health 2
411 - Association of Maternal Race and Ethnicity with Mother-Infant Dyad Outcomes in the Eating, Sleeping, Consoling for Neonatal Opioid Withdrawal (ESC-NOW) Randomized Controlled Trial
Saturday, April 26, 2025
2:30pm - 4:45pm HST
Publication Number: 411.3995
Kathryn Dee L. MacMillan, Children's Hospital at Dartmouth-Hitchcock, Lebanon, NH, United States; Zhuopei Hu, UAMS, Little Rock, AR, United States; Leslie W. Young, University of Vermont Larner College of Medicine, Burlington, VT, United States; Lori A.. Devlin, University of Louisville School of Medicine, Louisville, KY, United States; Stephanie L. Merhar, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States; Song Ounpraseuth, UAMS, Little Rock, AR, United States; Adrienne Pahl, The University of Vermont Children's Hospital, Burlington, VT, United States
- KM
Kathryn Dee L. MacMillan, MD MPH
Newborn Nursery Medical Director
Children's Hospital at Dartmouth-Hitchcock
Lebanon, New Hampshire, United States
Presenting Author(s)
Background: Maternal race affects access to medication for opioid use disorder (MOUD) and peripartum care. Limited studies suggest either no association between maternal race/ethnicity and outcomes for infants with neonatal opioid withdrawal syndrome (NOWS), or decreased pharmacotherapy among Black infants compared to white. Further characterization of this association is needed.
Objective: To evaluate associations between maternal race/ethnicity and NOWS hospital outcomes
Design/Methods: This secondary analysis included 1280 mother-infant dyads from the ESC-NOW study, a stepped-wedge cluster randomized controlled trial. All infants were 36 weeks’ gestation and managed for NOWS at one of 26 study hospitals. Outcomes of interest included receipt of pharmacologic treatment (PT), any breastmilk feeding during hospital stay (BF), and discharge with parents (DC).
A mixed-effect Poisson regression with robust error variance was used for binary outcomes. Relative risk was reported with 95% confidence intervals. Regression models accounted for trial design and were adjusted for site, maternal and infant characteristics. A sensitivity analysis removed infants with exposure to short-acting opioids for pain management.
Results: Infants categorized as non-Hispanic Black (n=169 (13%)), Hispanic (n=140 (11%)), or other (n=62 (5%)) were compared to those categorized as non-Hispanic white (n=904 (71%)) which served as the reference group. See Table 1 for maternal and infant characteristics.
In adjusted analyses, PT was less likely in non-Hispanic Black infants (RR 0.62, 95%CI:0.47-0.81). BF was less likely in Hispanic infants (RR 0.76, 95%CI:0.63-0.92). There was no statistically significant difference in DC with parents. PT remained less likely in Non-Hispanic Black infants in the sensitivity analysis (RR 0.66, 95% CI 0.51-0.85) (Table 2).
Conclusion(s): Maternal race/ethnicity were associated with differences in BF and PT. Infants born to Black mothers who used opioids during pregnancy were less likely to receive PT. This was true even after removing infants exposed to short-acting opioids frequently used for the management of pain in sickle cell disease and not exposed to maternal MOUD. Additionally, infants born to Hispanic mothers were less likely to receive any breastmilk during the birth hospitalization. These findings merit further exploration of systemic factors and potential bias in the management of infants with NOWS, and support for families during hospitalization.
Table 1. Baseline Characteristics of mothers and infants by Ethnicity and Race†
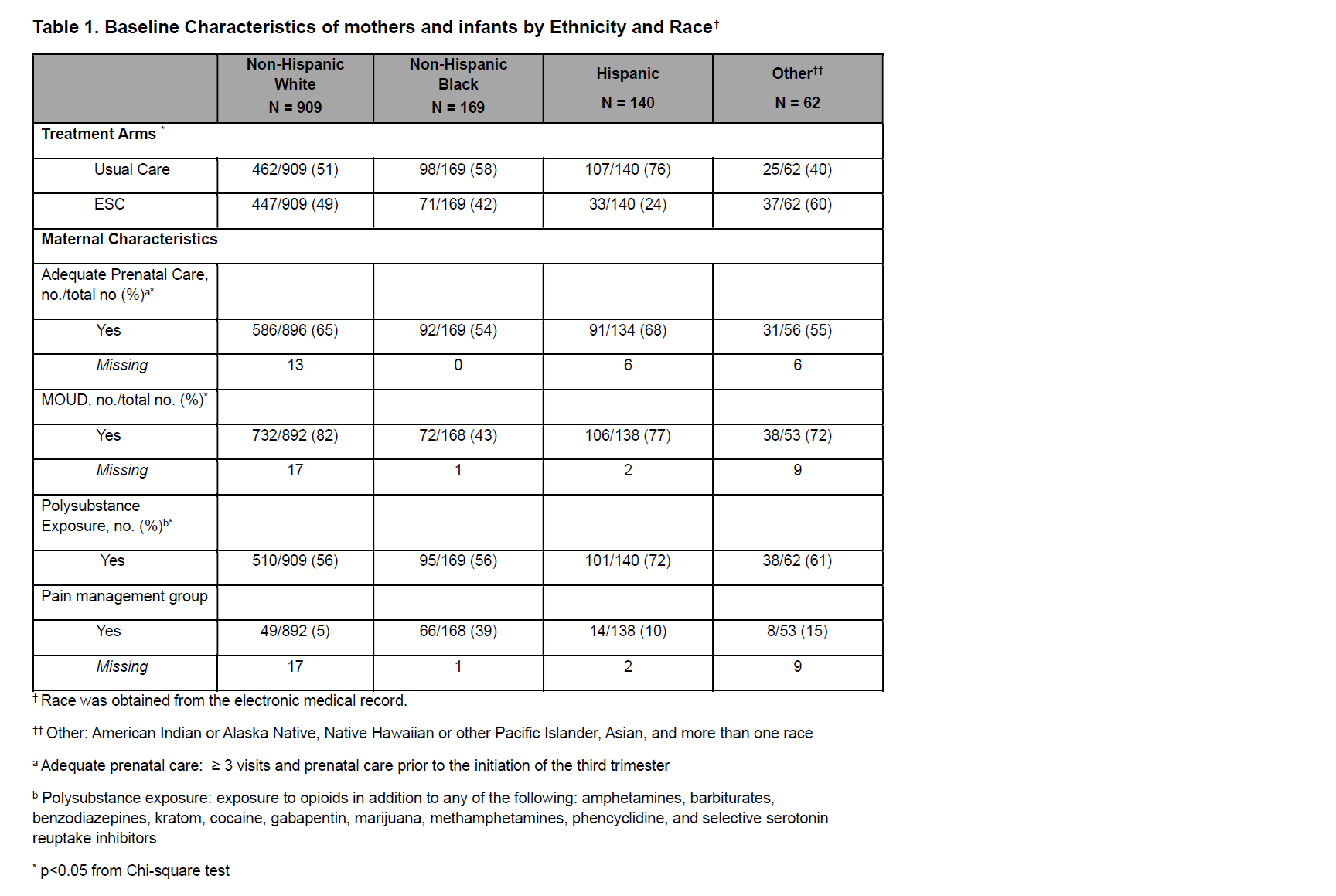
Table 2. Infant Hospital Outcomes by Maternal Race or Ethnicity
.png)
Table 3: Pharmacologic therapy subgroup analysis with pain management group removed
.png)

