Quality Improvement/Patient Safety 6
Session: Quality Improvement/Patient Safety 6
486 - Enhanced transparency and precision in reporting child health trial interventions using the Template for the Intervention Description and Replication checklist: TIDieR-Children & Adolescents
Saturday, April 26, 2025
2:30pm - 4:45pm HST
Publication Number: 486.4846
Katherine Goren, University of Toronto Temerty Faculty of Medicine, Toronto, ON, Canada; Veronica Ka Wai Lai, AdventHealth for Children, Saint John, NB, Canada; Ami Baba, The Hospital for Sick Children, Toronto, ON, Canada; Maureen Smith, The Hospital for Sick Children, Ottawa, ON, Canada; Tammy Hoffmann, Institute for Evidence-Based Healthcare, Bond University, Gold Coast, Queensland, Australia; Martin Offringa, The Hospital for Sick Children, Toronto, ON, Canada

Katherine Goren, MD (she/her/hers)
Paediatric Resident
University of Toronto Temerty Faculty of Medicine
Toronto, Ontario, Canada
Presenting Author(s)
Background: Pediatric randomized controlled trials (RCTs) inform clinical decisions affecting children and adolescents. To maximize the reproducibility and implementation of RCTs into clinical practice, complete and transparent reporting of both experimental and comparator trial interventions are needed.
Objective: To develop an evidence-informed, consensus-based, pediatric-specific reporting guideline for trial interventions highlighting key Pediatric Considerations and examples “Template for the Intervention Description and Replication (TIDieR) checklist: TIDieR-Children & Adolescents."
Design/Methods: Two researchers independently and in duplicate assessed intervention reporting against the TIDieR checklist in 50 pediatric RCTs published in 2022. They extracted examples of clear and complete reporting from trial texts. An international group of 20 pediatric clinical trialists and methodologists convened to discuss reporting gaps and generate Pediatric Considerations (PCs) around TIDieR items critical to transparent reporting. Participants provided good reporting examples from RCTs in their research areas. We ascertained caregiver input around PCs in a workshop with parents of children participating in RCTs. Pediatric Considerations (PCs) were amalgamated on pediatric-specific gaps identified. The international expert group then casted votes on a final list of PCs and illustrative examples. PCs and examples reaching >70% agreement were included in the final reporting guideline.
Results: The 50 RCTs encompassed a range of medical specialties (Table 1). Across all TIDieR items, experimental (e) interventions were better reported than comparators (c) (Figure 1). International experts yielded 14 PCs spanning TIDieR items #1-8 and #11. Good pediatric RCT reporting examples were found for both the experimental and comparator arms of each TIDieR item and corresponding PC. Table 2 features examples for three items with corresponding PCs and good reporting examples; the full list will be presented at the PAS meeting.
Conclusion(s): The TIDieR-Child & Adolescents checklist should improve the completeness of reporting, and ultimately the replicability and implementation of pediatric trial interventions.
Table 1. Pediatric randomized control trials characteristics
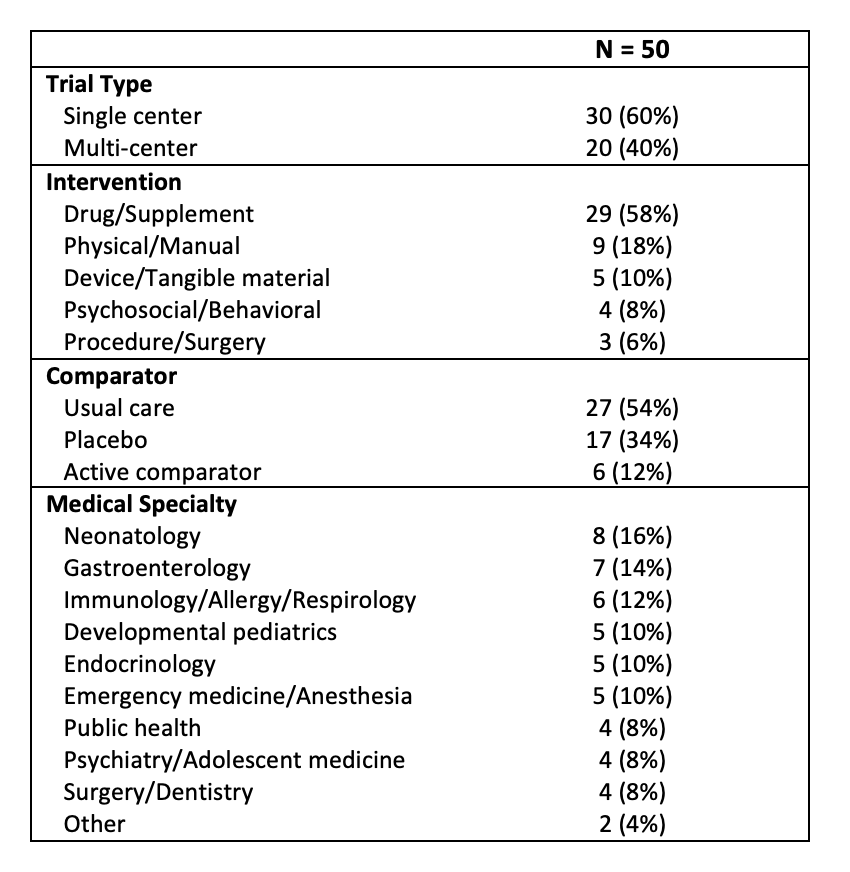
Table 2. TIDieR-C: example of original TIDieR item, pediatric considerations, and examples of good reporting for experimental and comparator trial interventions
.png)
Figure 1. Trial intervention reporting completeness in 50 pediatric randomized controlled trials, by TIDieR item
.png)

