Neonatal GI Physiology & NEC 4
Session: Neonatal GI Physiology & NEC 4
704 - Creatine Availability Bidirectionally Associated With Necrotizing Enterocolitis Pathogenesis
Monday, April 28, 2025
7:00am - 9:15am HST
Publication Number: 704.4409
Addison Franca, University of Oklahoma Health Sciences Center, Oklahoma City, OK, United States; Adam Wilson, Oklahoma Childrens Hospital at OU Health, Oklahoma City, OK, United States; Karni S. Moshal, University of Oklahoma College of Medicine, Oklahoma City, OK, United States; Hala Chaaban, Oklahoma University health sciences, Okl, OK, United States; Kathryn Burge, University of Oklahoma College of Medicine, Oklahoma City, OK, United States
- AF
Addison Franca-Faegre, B.S. Microbiology (she/her/hers)
Research Technician
University of Oklahoma Health Sciences Center
Oklahoma City, Oklahoma, United States
Presenting Author(s)
Background: Necrotizing enterocolitis (NEC) is a devastating inflammatory disorder of the preterm infant intestine. Contributing to NEC risk, the preterm ileum is characterized by inflammatory signaling, hypoxic episodes, and metabolic vulnerabilities. The well-known supplement, creatine (Cr), has profoundly beneficial effects on metabolism, promoting recycling of ATP, stimulating mitochondrial respiration, buffering tissues from hypoxic injury, and inhibiting apoptosis. Due to early delivery, preterm infants are likely deficient in Cr. In the adult colon, Cr supplementation stabilizes epithelial integrity, barrier function, and bioenergetics, improving disease outcomes. We hypothesize NEC development may accelerate Cr tissue depletion, while Cr supplementation will improve bioenergetic potential.
Objective: Delineate the impact of Cr supplementation on preterm ileal enteroid bioenergetics and explore the potential association of Cr tissue depletion with NEC pathogenesis.
Design/Methods: An XF Real-Time ATP Rate Assay was run using a Seahorse XF HS Mini instrument. Preterm enteroid-derived single-cell suspensions were plated on an XF HS Miniplate using an open-faced sandwich method. Enteroids were treated with 10 mM Cr or vehicle control (n=3 wells/group) for 24 h. 1.5 μM oligomycin and 0.5 μM rotenone/antimycin A were used to evaluate mitochondrial and glycolytic ATP synthesis, respectively, and cell numbers were normalized to DNA content. To investigate a potential relationship between preterm NEC pathogenesis and tissue Cr depletion, ileal surgical resections from infants with modified Bell’s Stage IIB+ NEC and control (e.g., intestinal atresia), as well as longitudinal urine samples from infants with NEC, were acquired. Tissue levels of Cr, phospho-Cr, and associated molecules will be assessed using high-resolution metabolomics.
Results: 10 mM Cr supplementation significantly increased preterm enteroid mitochondrial (Fig. 1A/C; p< 0.0003) and glycolytic (Fig. 1B/D; p< 0.0001) ATP synthesis compared to control. Ileal resections (n=3 NEC, n=2 control) and longitudinal urine samples from preterm infants with NEC (n=9) have been acquired. Metabolomics data is pending.
Conclusion(s): Cr improves mitochondrial bioenergetic capacity within the preterm epithelium, potentially providing an energetic buffer with which to combat prematurity-associated intestinal hypoxia and inflammation. Future studies may provide rationale for exploration of Cr supplementation as a prophylactic therapy for infants at risk for NEC development.
Figure 1
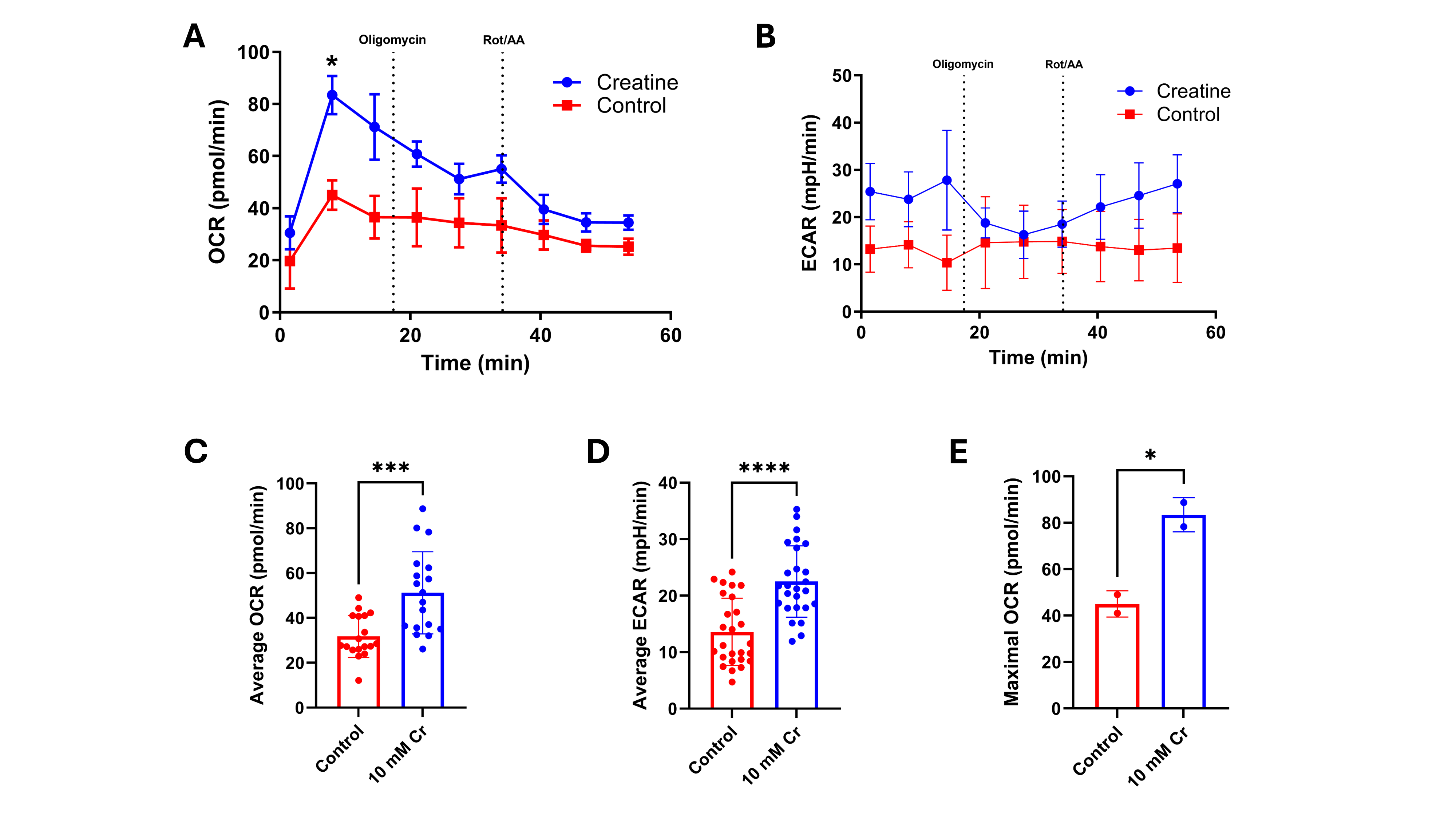 24h Cr (10 mM) treatment of preterm ileal enteroids increases metabolic capacity. Cr significantly increased OCR (A&D-E) and ECAR, but not at a specific timepoint (B&E); n=2-3 wells/treatment. A&B: *p=0.0291/*p=0.0136, p=0.6401/p=0.0621 by 2-way ANOVA for effect of time/treatment, respectively. C-E: p=0.0003, p<0.0001, p=0.0278 by student’s t-test.
24h Cr (10 mM) treatment of preterm ileal enteroids increases metabolic capacity. Cr significantly increased OCR (A&D-E) and ECAR, but not at a specific timepoint (B&E); n=2-3 wells/treatment. A&B: *p=0.0291/*p=0.0136, p=0.6401/p=0.0621 by 2-way ANOVA for effect of time/treatment, respectively. C-E: p=0.0003, p<0.0001, p=0.0278 by student’s t-test.
